Abstract
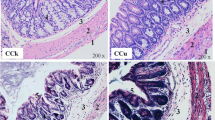
Twelve Kunming mice were randomly divided into two groups (n = 6), and administered with distilled water containing 0 mg/L and 160 mg/L HgCl2 respectively, with an experimental period of 3 days. Our results showed that mercury exposure significantly reduced weight gain in mice (P < 0.01). Through pathological observation of cecum tissues, significant pathological changes were observed in cecum tissues of mice exposed to mercury. Furthermore, mercury exposure not only significantly increased malondialdehyde (MDA) content in mice (P < 0.01) but also significantly decreased superoxide dismutase (SOD) activity (P < 0.01) and glutathione peroxidase (GSH) level in mice (P < 0.01). Furthermore, high-throughput sequencing analysis showed that at the genus level some microbial populations including Clostridiales, Lactobacillus, Treponema, Oscillospira, and Desulfovibrio were significantly increased whereas some microbial populations including S24-7, Acinetobacter, and Staphylococcus were significantly decreased. Moreover, correlation analysis indicated that microorganisms were not correlated with biomarkers of oxidative stress. In summary, mercury exposure reduced the growth performance of mice, resulting in gut microbiota alterations, and led to oxidative stress by increasing the concentration of malondialdehyde (MDA) and decreasing the concentration of superoxide dismutase (SOD) and glutathione peroxidase (GSH).







Similar content being viewed by others
Abbreviations
- MDA:
-
malondialdehyde
- GSH:
-
glutathione peroxidase
- SOD:
-
superoxide dismutase
- WHO:
-
World Health Organization
References
Liu J, Xu X, Yu S, Cheng H, Hong Y, Feng X (2014) Mercury pollution in fish from South China Sea: levels, species-specific accumulation, and possible sources. Environ Res 131:160–164. https://doi.org/10.1016/j.envres.2014.03.004
Jiang GB, Shi JB, Feng XB (2006) Mercury pollution in China. An overview of the past and current sources of the toxic metal. Environ Sci Technol 40(12):3673–3678. https://doi.org/10.1021/es062707c
de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovsky Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A (2006) Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect 114(4):584–590. https://doi.org/10.1289/ehp.8202
Li S, Baiyun R, Lv Z, Li J, Han D, Zhao W, Yu L, Deng N, Liu Z, Zhang Z (2019) Exploring the kidney hazard of exposure to mercuric chloride in mice:Disorder of mitochondrial dynamics induces oxidative stress and results in apoptosis. Chemosphere 234:822–829. https://doi.org/10.1016/j.chemosphere.2019.06.096
Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q, Zhang X, Zhang Z (2018) Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: involvement of AKT/Nrf2 and NF-kappaB pathways. Food Chem Toxicol 113:296–302. https://doi.org/10.1016/j.fct.2018.02.003
Bollengier-Lee S, Mitchell MA, Utomo DB, Williams PE, Whitehead CC (1998) Influence of high dietary vitamin E supplementation on egg production and plasma characteristics in hens subjected to heat stress. Br Poult Sci 39(1):106–112. https://doi.org/10.1080/00071669889466
Yuan M, Li D, Zhang Z, Sun H, An M, Wang G (2018) Endometriosis induces gut microbiota alterations in mice. Hum Reprod 33(4):607–616. https://doi.org/10.1093/humrep/dex372
Rueda-Ruzafa L, Cruz F, Roman P, Cardona D (2019) Gut microbiota and neurological effects of glyphosate. Neurotoxicology 75:1–8. https://doi.org/10.1016/j.neuro.2019.08.006
Kou H, Fu Y, He Y, Jiang J, Gao X, Zhao H (2019) Chronic lead exposure induces histopathological damage, microbiota dysbiosis and immune disorder in the cecum of female Japanese quails (Coturnix japonica). Ecotoxicol Environ Saf 183:109588. https://doi.org/10.1016/j.ecoenv.2019.109588
Ruan Y, Wu C, Guo X, Xu Z, Xing C, Cao H, Zhang C, Hu G, Liu P (2019) High doses of copper and mercury changed cecal microbiota in female mice. Biol Trace Elem Res 189(1):134–144. https://doi.org/10.1007/s12011-018-1456-1
Skrypnik K, Suliburska J (2018) Association between the gut microbiota and mineral metabolism. J Sci Food Agric 98(7):2449–2460. https://doi.org/10.1002/jsfa.8724
Skrypnik K, Bogdanski P, Sobieska M, Suliburska J (2020) Hepcidin and erythroferrone correlate with hepatic Iron transporters in rats supplemented with multispecies probiotics. Molecules 25(7):1674. https://doi.org/10.3390/molecules25071674
Li S, Jiang X, Luo Y, Zhou B, Shi M, Liu F, Sha A (2019) Sodium/calcium overload and Sirt1/Nrf2/OH-1 pathway are critical events in mercuric chloride-induced nephrotoxicity. Chemosphere 234:579–588. https://doi.org/10.1016/j.chemosphere.2019.06.095
Skrypnik K, Bogdański P, Schmidt M, Suliburska J (2019) The effect of multispecies probiotic supplementation on iron status in rats. Biol Trace Elem Res 192(2):234–243. https://doi.org/10.1007/s12011-019-1658-1
Sutton DJ, Tchounwou PB (2007) Mercury induces the externalization of phosphatidyl-serine in human renal proximal tubule (HK-2) cells. Int J Environ Res Public Health 4(2):138–144. https://doi.org/10.3390/ijerph2007040008
Pal PB, Pal S, Das J, Sil PC (2012) Modulation of mercury-induced mitochondria-dependent apoptosis by glycine in hepatocytes. Amino Acids 42(5):1669–1683. https://doi.org/10.1007/s00726-011-0869-3
Ansaldo M, Najle R, Luquet CM (2005) Oxidative stress generated by diesel seawater contamination in the digestive gland of the Antarctic limpet Nacella concinna. Mar Environ Res 59(4):381–390. https://doi.org/10.1016/j.marenvres.2004.06.003
Troost FJ, Saris WH, Haenen GR, Bast A, Brummer RJ (2003) New method to study oxidative damage and antioxidants in the human small bowel: effects of iron application. Am J Physiol Gastrointest Liver Physiol 285(2):G354–G359. https://doi.org/10.1152/ajpgi.00422.2002
McCord JM, Fridovich I (1968) The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 243(21):5753–5760
Deng Q, Xu J, Yu B, He J, Zhang K, Ding X, Chen D (2010) Effect of dietary tea polyphenols on growth performance and cell-mediated immune response of post-weaning piglets under oxidative stress. Arch Anim Nutr 64(1):12–21. https://doi.org/10.1080/17450390903169138
Kawauchiya T, Takumi R, Kudo Y, Takamori A, Sasagawa T, Takahashi K, Kikuchi H (2011) Correlation between the destruction of tight junction by patulin treatment and increase of phosphorylation of ZO-1 in Caco-2 human colon cancer cells. Toxicol Lett 205(2):196–202. https://doi.org/10.1016/j.toxlet.2011.06.006
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47(2):74–83. https://doi.org/10.3961/jpmph.2014.47.2.74
Zhang BB, Liu YM, Hu AL, Xu SF, Fan LD, Cheng ML, Li C, Wei LX, Liu J (2019) HgS and Zuotai differ from HgCl2 and methyl mercury in intestinal Hg absorption, transporter expression and gut microbiome in mice. Toxicol Appl Pharmacol 379:114615. https://doi.org/10.1016/j.taap.2019.114615
Ye F, Li X, Li L, Lyu L, Yuan J, Chen J (2015) The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem Toxicol 86:191–201. https://doi.org/10.1016/j.fct.2015.10.009
El-Saeed GSM, Abdel Maksoud SA, Bassyouni HT, Raafat J, Agybi MH, Wahby AA, Aly HM (2016) Mercury toxicity and DNA damage in patients with Down syndrome. Med Res J 15(1):22–26. https://doi.org/10.1097/01.MJX.0000483973.37399.e7
Yang J, Zhang X, Xie Y, Song C, Sun J, Zhang Y, Giesy JP, Yu H (2017) Ecogenomics of zooplankton community reveals ecological threshold of ammonia nitrogen. Environ Sci Technol 51(5):3057–3064. https://doi.org/10.1021/acs.est.6b05606
Funding
This project was supported by the National Natural Science Foundation of China grant (no. 31960723; 31460679 Beijing, P. R. China) awarded to PL and the Natural Science Foundation of Jiangxi Province grant (no. 20171ACB21026; 2017ACB20012) awarded to PL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yulan Zhao and Changming Zhou are equal first author.
Rights and permissions
About this article
Cite this article
Zhao, Y., Zhou, C., Guo, X. et al. Exposed to Mercury-Induced Oxidative Stress, Changes of Intestinal Microflora, and Association between them in Mice. Biol Trace Elem Res 199, 1900–1907 (2021). https://doi.org/10.1007/s12011-020-02300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02300-x