Abstract
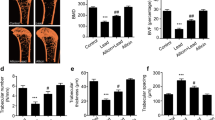
The aim of this study was to investigate the long-term effects of low-dose lead exposure on bone microstructure in mice. Ten SPF 12-week-old male C57BL/6J mice were randomly divided into two groups: control (deionized water) and lead exposure (150 ppm of lead acetate in drinking water). After 24 weeks treatment, mice were weighed and the left femurs were collected and stored at − 80 °C. The right femurs of the mice were scanned by Micro-CT for three-dimensional reconstruction, and bone mineral density, bone volume fraction, trabeculae thickness, trabeculae number, and trabeculae separation were measured. The right tibia was collected to investigate histopathological changes in H&E-stained sections. The gene expression of osteoprotegerin (OPG), RANKL, and runt-related transcription factor 2 (Runx2) was determined using real-time PCR. The bone density of femoral cancellous bone and the number of cancellous bone trabeculae in the lead exposure group were both significantly decreased compared with the control group. Bone marrow stromal cell numbers were decreased following lead administration, and lipid droplet vacuoles were observed in the lead group. Levels of OPG were significantly decreased in the lead group, and lead also inhibited the expression of Runx2 compared with the control group. Long-term exposure to low doses of lead can cause bone damage without inducing other obvious symptoms through decreasing bone density and the number of cancellous bone trabeculae, further suppressing bone formation. It suggests that lead may exacerbate bone loss and osteoporosis, especially in the elderly.







Similar content being viewed by others
References
Tong S, Schirnding YE, Von PT (2000) Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ 78(9):1068
Kasperczyk A, Dobrakowski M, Czuba ZP, Horak S, Kasperczyk S (2015) Environmental exposure to lead induces oxidative stress and modulates the function of the antioxidant defense system and the immune system in the semen of males with normal semen profile. Toxicol Appl Pharmacol 284(3):339–344
Wildemann TM, Mirhosseini N, Siciliano SD, Weber LP (2015) Cardiovascular responses to lead are biphasic, while methylmercury, but not inorganic mercury, monotonically increases blood pressure in rats. Toxicology 328:1–11
Murata K, Araki S (2010) Autonomic nervous system dysfunction in workers exposed to lead, zinc, and copper in relation to peripheral nerve conduction: a study of R-R interval variability. Am J Ind Med 20(5):663–671
Caito S, Acba L, Mmb P, Aschner M (2017) Toxicology of lead and its damage to mammalian organs. Metal Ions in Life Sciences 17
Parfitt AM (1988) Bone histomorphometry: standardization of nomenclature, symbols and units (summary of proposed system). Bone 9(1):67–69
Polák J, O’Flaherty EJ (1995) Physiologically based models of lead exposure in children. Toxicol Lett 78(4):67
Hu H, Rabinowitz M, Smith D (1998) Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect 106(1):1–8
Skerfving S, Nilsson U (1992) Assessment of accumulated body burden of metals. Toxicology letters 64-65 spec no:17
Gamblin C, Gordon CL, Muir DCF, Chettle DR, Webber CE (1994) In vivo measurements of bone lead content in residents of Southern Ontario. Appl Radiat Isot 45(10):1035–1038
Berlin K, Gerhardsson L, Börjesson J, Lindh E, Lundström N, Schütz A, Skerfving S, Edling C (1995) Lead intoxication caused by skeletal disease. Scand J Work Environ Health 21(4):296–300
Potula V, Henderson A, Kaye W (2005) Calcitropic hormones, bone turnover, and lead exposure among female smelter workers. Arch Environ Occup Health 60(4):195–204
Beier EE, Maher JR, Sheu TJ, Cory-Slechta DA, Berger AJ, Zuscik MJ, Puzas JE (2012) Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ Health Perspect 121(1):97–104
Beier EE, Inzana JA, Sheu TJ, Shu L, Puzas JE, Mooney RA (2015) Effects of combined exposure to lead and high-fat diet on bone quality in juvenile male mice. Environ Health Perspect 123(10):935–943. https://doi.org/10.1289/ehp.1408581
Campbell JR, Rosier RN, Novotny L, Puzas JE (2004) The association between environmental lead exposure and bone density in children. Environ Health Perspect 112(11):1200–1203. https://doi.org/10.1289/ehp.6555
Beier EE, Holz JD, Sheu TJ, Puzas JE (2016) Elevated lifetime lead exposure impedes osteoclast activity and produces an increase in bone mass in adolescent mice. Toxicol Sci : Off J Soc Toxicol 149(2):277–288. https://doi.org/10.1093/toxsci/kfv234
Bleecker ML, McNeill FE, Lindgren KN, Masten VL, Ford DP (1995) Relationship between bone lead and other indices of lead exposure in smelter workers. Toxicol Lett 77(1–3):241–248
Wedeen RP, Maesaka JK, Weiner B, Lipat GA, Lyons MM, Vitale LF, Joselow MM (1975) Occupational lead nephropathy. Am J Med 59(5):630–641
Karimooy HN, Mood MB, Hosseini M, Shadmanfar S (2010) Effects of occupational lead exposure on renal and nervous system of workers of traditional tile factories in Mashhad (northeast of Iran). Toxicol Ind Health 26(9):633–638
Evans M, Fored CM, Nise G, Bellocco R, Nyrén O, Elinder CG (2010) Occupational lead exposure and severe CKD: a population-based case-control and prospective observational cohort study in Sweden. Am J Kidney Dis 55(3):497–506
C C (1985) Preventing lead poisoning in young children--United States. MMWR Morb Mortal Wkly Rep 34 (5):66–73
Campbell JR, Auinger P (2007) The association between blood lead levels and osteoporosis among adults: results from the Third National Health and Nutrition Examination Survey (NHANES III). Environ Health Perspect 115(7):1018–1022
Khalil N, Cauley JA, Wilson JW, Talbott EO, Morrow L, Hochberg MC, Hillier TA, Muldoon SB, Cummings SR (2008) Relationship of blood lead levels to incident nonspine fractures and falls in older women: the study of osteoporotic fractures. J Bone Miner Res 23(9):1417–1425. https://doi.org/10.1359/jbmr.080404
Á-L P, Lee CM, Conti MI, Terrizzi AR, González-López S, Martínez MP (2017) Effects of chronic lead exposure on bone mineral properties in femurs of growing rats. Toxicology 377
Carmouche JJ, Puzas JE, Zhang X, Tiyapatanaputi P, Coryslechta DA, Gelein R, Zuscik M, Rosier RN, Boyce BF, O’Keefe RJ (2005) Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspect 113(6):749–755
Ashton JR, West JL, Badea CT (2015) In vivo small animal micro-CT using nanoparticle contrast agents. Front Pharmacol 6:256. https://doi.org/10.3389/fphar.2015.00256
Van RB, Majumdar S, Pistoia W, Newitt DC, Kothari M, Laib A, Rüegsegger P (1998) Assessment of cancellous bone mechanical properties from micro-FE models based on micro-CT, pQCT and MR images. Technol Health Care Off J Eur Soc Eng Med 6(5–6):413–420
Res JBM (2010) Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. Journal of Bone & Mineral Research the Official Journal of the American Society for Bone & Mineral Research 14(12):2159–2166
Alvarez-Lloret P, Lee CM, Conti MI, Terrizzi AR, Gonzalez-Lopez S, Martinez MP (2017) Effects of chronic lead exposure on bone mineral properties in femurs of growing rats. Toxicology 377:64–72. https://doi.org/10.1016/j.tox.2016.11.017
Yuan LQ, Zhu JH, Wang HW, Liang QH, Xie H, Wu XP, Zhou H, Cui RR, Sheng ZF, Zhou HD (2011) RANKL is a downstream mediator for insulin-induced osteoblastic differentiation of vascular smooth muscle cells. Bone 47(12):S452–S452
Bezerra MC, Carvalho JF, Prokopowitsch AS, Pereira RMR (2005) RANK, RANKL and osteoprotegerin in arthritic bone loss. Braz J Med Biol Res 38(2):161–170
Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E (1999) Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor. Bone 25(5):517–523
Thomas GP, Baker SU, Eisman JA, Gardiner EM (2001) Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol 170(2):451–460
Monir AU, Gundberg CM, Yagerman SE, van der Meulen MC, Budell WC, Boskey AL, Dowd TL (2010) The effect of lead on bone mineral properties from female adult C57/BL6 mice. Bone 47(5):888–894. https://doi.org/10.1016/j.bone.2010.07.013
Lei J, Liu MQ, Yap AUJ, Fu KY (2012) Condylar subchondral formation of cortical bone in adolescents and young adults. Br J Oral Maxillofac Surg 51(1):63–68
Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M (2018) High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res: Off J Am Soc Bone Miner Res 33(6):1154–1165. https://doi.org/10.1002/jbmr.3408
Funding
This work was supported by National Natural Science Foundation of China (grant number 81773414) and National Natural Science Foundation of China (grant number 81673151).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care followed the Guide for the Care and Use of Laboratory Animal, and the study protocols were approved by the Soochow University Institutional Animal Care and Use Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheng, Z., Wang, S., Zhang, X. et al. Long-Term Exposure to Low-Dose Lead Induced Deterioration in Bone Microstructure of Male Mice. Biol Trace Elem Res 195, 491–498 (2020). https://doi.org/10.1007/s12011-019-01864-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01864-7