Abstract
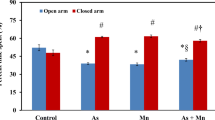
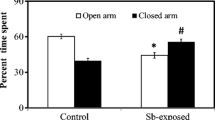
Arsenic (As) toxicity has caused an environmental tragedy affecting millions of people in the world. Little is known about the toxic effects of As on neurobehavioral and biochemical changes in vivo. Along this line of metal toxicity, co-exposure of lead (Pb) could aggravate the situation in the host. The present study was designed to explore the combined effects of As and Pb on behavioral changes like anxiety, spatial memory and learning impairment, and blood indices related to organ dysfunction. Exposure of mice to As (10 mg/kg body weight), Pb (10 mg/kg body weight), and As + Pb via drinking water significantly decreased the time spent exploring the open arms while it increased the time spent in the closed arms compared to control mice in the elevated plus maze. The mean latency time of the control group to find the platform decreased significantly during the learning for 7 days compared to all three treated groups in the Morris water maze test, and the As-exposed group spent significantly less time in the desired quadrant as compared to the control group in the probe trial. Both metals posed an anxiety-like behavior and deficits in spatial memory and learning, and also altered blood indices related to liver and kidney dysfunction, and a combined exposure of these metals inhibited the individual accumulation of As and Pb. Taken together, these data suggest that As has more toxic effects on neurobehavioral and biochemical changes than Pb, and there may be antagonism in the effects and accumulation between these two toxicants.


Similar content being viewed by others
References
Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, van Geen A, Graziano J, Ahsan H (2010) Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376:252–258
Chowdhury UK, Biswas BK, Chowdhury TR (2000) Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect 108(5):393–397
Tapio S, Grosche B (2006) Arsenic in the aetiology of cancer. Mutat Res 612:215–246
GuhaMazumder DN, Haque R, Ghosh N, De BK, Santra A, Chakraborty D, Smith AH (1998) Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int J Epidemiol 27(5):871–877
Parvez F, Chen Y, Maria A, Hussain AI, Hassina M, Dhar R, Geen A, Graziano J, Ahsan H (2006) Prevalence of arsenic exposure from drinking water and awareness of its health risks in a Bangladeshi population; results from a large population-based study. Environ Health Perspect 114(3):355–359
Christina RT, Andrea MA (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 1:132–147
Ozone K, Ueno S, Ishizaki M, Hayashi O (2000) Toxicity and oxidative stress induced by organic arsenical diphenylarsinic acid and inorganic arsenicals and their effects on spatial learning ability in mice. J Health Sci 56:517–526
Hei TK, Liu SX, Waldren C (1998) Mutagenicity of arsenic in mammalian cells; role of reactive oxygen species. Proc Natl Acad Sci 95(14):8103–8107
Liu SX, Athar M, Lippai I, Waldren C, Hei TK (2001) Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci 98(4):1643–1648
Lynn S, Shiung JN, Gurr JR, Jan KY (1998) Arsenic stimulates poly (ADP ribosylation) by generation of nitric oxide. Free Rad Biol Med 24(3):442–449
Frisbie SH, Ortega R, Maynard DM, Sarkar B (2002) The concentrations of arsenic and other toxic elements in Bangladesh’s drinking water. Environ Health Perspect 110(11):1147–1153
Englyst V, Lundstrom NG, Gerhandsson L, Rylander L, Nordberg G (2001) Lung cancer risks among lead smelter workers also exposed to arsenic. Sci Total Environ 273:77–82
Nehez M, Lorencz R, Desi I (2000) Simultaneous action of cypermethrin and two environmental pollutant metals, cadmium and lead, on bone marrow cell chromosomes of rats in subchronic administration. Ecotoxicol Environ Saf 45(1):55–60
Elias RW (1985) Lead exposure in human environment. In: Mahaffey K (ed) Dietary and environmental lead. Human health effects. Elsevier, Amsterdam-New York-Oxford, pp. 79–107
Lambert TW, Lane S (2004) Lead, arsenic and polycyclic aromatic hydrocarbons in soil and house dust in the communities sorrounding the Sydney, Nova Scotia, tar ponds. Environ Health Perspect 112(1):35–41
Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, Phillips RS (2004) Heavy metal content of Ayurvedic herbal medicine products. JAMA 292(23):2868–2873
Hoffer BJ, Olson L, Palmer MR (1987) Toxic effects of lead in the developing nervous system: in oculo experimental models. Environ Health Perspect 74:169–175
WHO (2010) Childhood lead poisoning. WHO, Geneva, Switzerland
Antonio MT, Corredor L, Leret ML (2003) Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol Lett 143(3):331–340
Lanphear BP, Hornung R, Ho M, Howard CR, Eberle S, Knauf K (2002) Environmental lead exposure during early childhood. J Pediatr 140(1):40–47
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM (2009) Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol 239(2):169–177
Correa M, Roig-Navarro AF, Aragon CM (2004) Motor behavior and brain enzymatic changes after acute lead intoxication on different strains of mice. Life Sci 74(16):2009–2021
Rodriguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M (2002) Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol 24(6):743–750
Calderón J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V, Díaz-Barriga F (2001) Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res 85(2):69–76
Ramírez-Campos J, Ramos-Peek J, Martínez-Barros M, Zamora-Peralta M, Martínez-Cerrato J (1998) Peripheral neuropathy caused by acute arsenic poisoning. Gac Med Mex 134(2):241–246
Bishayi B, Sengupta M (2006) Synergism in immune toxicological effects due to repeated combined administration of arsenic and lead in mice. Int Immunopharmacol 6(3):454–464
Saritha S, Kumar PK, Sreenivasula PR, Tripathy NK, Rajarami GR (2014) Developmental arsenic and lead exposure: behavioral and neurochemical perturbations of albino rats. IAJPR 4:1707–1716
Das AP, Bag S, Sahu R, Dua TK, Sinha MK, Gangopadhyay M, Zaman K, Dewanjee S (2010) Protective effect of Corchorus olitorius leaves on sodium arsenite-induced toxicity in experimental rats. Food Chem Toxicol 48:326–335
Rodriguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M (2001) The effect of sodium arsenite exposure on behavioral parameters in rat. Brain Res Bull 55(2):301–308
Karim MR, Haque A, Islam K, Ali N, Salam KA, Saud ZA, Hossain E, Fajol A, Akhand AA, Himeno S, Hossain K (2010) Protective effects of the dietary supplementation of turmeric (Curcuma longa L.) on sodium arsenite-induced biochemical perturbation in mice. Bangladesh Med Res Council Bull 36:82–88
Xu Y, Li G, Han C, Sun L, Zhao R, Cui S (2005) Protective effects of Hippophae rhamnoides L. juice on lead-induced neurotoxicity in mice. Biol Pharm Bull 28(3):490–494
Mishra M, Acharya UR (2004) Protective action of vitamins on the spermatogenesis in lead-treated Swiss mice. J Trace Element in Med and Biol 18:173–178
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open-closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Leret ML, Millan JAS, Antonio MT (2003) Perinatal exposure to lead and cadmium affects anxiety-like behavior. Toxicology 186:125–130
D'Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36(1):60–90
Ali N, Hoque MA, Haque A, Salam KA, Karim MR, Rahman A, Islam K, Saud ZA, Khalek MA, Akhand AA, Hossain M, Mandal A, Karim MR, Miyataka H, Himeno S, Hossain K (2010) Association between arsenic exposure and plasma cholinesterase activity: a population based study in Bangladesh. Environ Health 9:36
Tuot DS, Plantinga LC, Hsu CY, Jordan R, Burrows NR, Hedgeman E, Yee J, Saran R, Powe NR, Centers for Disease Control Chronic Kidney Disease Surveillance Team (2011) Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol 6(8):1838–1844
Wang JP, Wang SL, Lin Q, Zhang L, Huang D, Nq JC (2009) Association of arsenic and kidney dysfunction in people with diabetes and validation of its effects in rats. Environ Int 35(3):507–511
Islam MS, Mohanto NC, Karim MR, Aktar S, Hoque MM, Rahman A, Jahan M, Khatun R, Aziz A, Salam KA, Saud ZA, Hossain M, Rahman A, Mandal A, Haque A, Miyataka H, Himeno S, Hossain K (2015) Elevated concentrations of serum matrix metalloproteinase-2 and -9 and their associations with circulating markers of cardiovascular diseases in chronic arsenic-exposed individuals. Environ Health 14:92
Karim MR, Rahman M, Islam MAA, Hossain S, Hossain E, Aziz A, Yeasmin F, Agarwal S, Hossain MI, Saud ZA, Nikkon F, Hossain M, Mandal A, Jenkins RO, Haris PI, Miyataka H, Himeno S, Hossain K (2013) Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicol Sci 135:17–25
Vahidnia A, Romijn F, van derVoet GB, de Wolff FA (2008) Arsenic-induced neurotoxicity in relation to toxicokinetics: effects on sciatic nerve proteins. Chem Biol Interact 176:188–195
Finkelstein Y, Markowitz ME, Rosen JF (1998) Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev 27(2):168–176
Abazyan B, Dziedzic J, Hua K, Abazyan S, Yang C, Mori S, Pletnikov MV, Guilarte TR (2014) Chronic exposure of mutant DISC1 mice to lead produces sex-dependent abnormalities consistent with schizophrenia and related mental disorders: a gene-environment interaction study. Schizophr Bull 40(3):575–584
Gross C, Hen R (2004) The developmental origins of anxiety. Nat Rev Neurosci 5(7):545–552
Weinberger DR (2001) Anxiety at the frontier of molecular medicine. N Engl J Med 344(16):1247–1249
Belzung C, Griebel G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125:141–149
Kahloula K, Slimani M, Aoues A (2009) Behavioural and neurochemical studies of perinatal lead exposed in rat wistar. Eur J Sci Res 35:603–614
Brinkel J, Khan MMH, Kraemer A (2008) A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Res Public Health 6(5):1609–1619
Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Caamano MC, Cebrian ME, Stoltzfus R (2007) Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect 115(9):1371–1375
Von Ehrenstein OS, Poddar S, Yuan Y, Mazumder DG, Eskenazi B, Basu A, Hira-Smith M, Ghosh N, Lahiri S, Haque R, Ghosh A, Kalman D, Das S, Smith AH (2007) Children’s intellectual function in relation to arsenic exposure. Epidemiology 18(1):44–51
Ramos-Chávez LA, Rendón-López CRR, Zepeda A, Silva-Adaya D, Del Razo LM, Gonsebatt ME (2015) Neurological effects of inorganic arsenic exposure: altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front Cell Neurosci 9:21
Gandhi DN, Kumar R (2013) Arsenic toxicity and neurobehaviors: a review. Innovation Pharma Pharmacotherapy 1:1–15
Bokara KK, Brown E, McCormick R, Yallapragada PR, Rajanna S, Bettaiya R (2008) Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. Biometals 21(1):9–16
Ercal N, Treeratphan P, Hammond TC, Matthews RH, Grannemann NH, Spitz DR (1996) In vivo indices of oxidative stress in lead exposed C57BL/6 mice are reduced by treatment with meso-2,3-dimercaptosuccinic acid or N-acetylcysteine. Free Radic Biol Med 21(2):157–161
Fortune T, Lurie DI (2009) Chronic low level lead exposure affects the monoaminergic system in the mouse superior olivary complex. J Comp Neurol 513(5):542–558
Kala SV, Jadhav AL (1995) Region-specific alterations in dopamine and serotonin metabolism in brains of rats exposed to low levels of lead. Neurotoxicology 16(2):297–308
Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK (2002) Glutathione, iron and Parkinson’s disease. Biochem Pharmacol 64(5–6):1037–1048
Dringen R, Gutterer JM, Hirrlinger J (2002) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267(16):4912–4916
Rahman M, Saud ZA, Hossain E, Islam K, Karim MR, Yeasmin T, Nikkon F, Mandal A, Hossain K (2012) Ameliorating effects of Zingiber zerumbet Linn. on sodium arsenite-induced changes of blood indices in experimental mice. Life Sci Med Res:41
Oyeronke AO, Kazeem AA, Babatunde O, Oladimeji T (2007) Interaction and enhancement of the toxic effects of sodium arsenite and lead acetate in Wistar rats. Afr J Biomed Res 10:59–65
Eisenbach C, Sieg O, Stremmel W, Encke J, Merle U (2007) Diagnostic criteria for acute liver failure due to Wilson disease. World J Gastroenterol 13(11):1711–1714
Kaniaris P, Fassoulaki A, Liarmakopoulou K, Dermitzakis E (1979) Serum cholinesterase levels in patients with cancer. Anesth Analg 58:82–84
Montenegro MF, Ruiz-Espejo F, Campoy FJ, Muñoz-Delgado E, de la Cadena MP, Cabezas-Herrera J, Vidal CJ (2006) Acetyl- and butyrylcholinesterase activities decrease in human colon adenocarcinoma. J Mol Neurosci 30(1–2):51–54
Patlolla AK, Tchounwou PB (2005) Serum acetyl cholinesterase as a biomarker of arsenic induced neurotoxicity in Sprague-Dawley rats. Int J Environ Res Public Health 2(1):80–83
Geula C, Darvesh S (2004) Butyrylcholinesterase, cholinergic neurotransmission and the pathology of Alzheimer’s disease. Drugs Today (Barc) 40(8):711–721
Flora SJ, Mittal M, Mishra D (2009) Co-exposure to arsenic and fluoride on oxidative stress, glutathione linked enzymes, biogenic amines and DNA damage in mouse brain. J Neurol Sci 285(1–2):198–205
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology, Government of People’s Republic of Bangladesh (39.009.006.01.00.049.2013-2014/BS/104), and the University of Rajshahi (300(6)-5/52/RABI/BINGAN (1)/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Aktar, S., Jahan, M., Alam, S. et al. Individual and Combined Effects of Arsenic and Lead on Behavioral and Biochemical Changes in Mice. Biol Trace Elem Res 177, 288–296 (2017). https://doi.org/10.1007/s12011-016-0883-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0883-0