Abstract
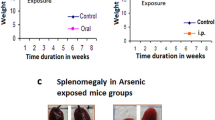
Exposure to arsenic on a regular basis, mainly through drinking water, agricultural pesticide, and sometimes therapeutic dose, results in various diseases of different tissues including the bone marrow hematopoietic system. Hematopoiesis is a dynamic process by which bone marrow (BM) hematopoietic stem/progenitor cells (HSPCs) generate a relatively constant pool of functionally mature blood cells by the support of microenvironmental components. The present study has been aimed to understand stem cell microenvironmental status during arsenic toxicity and the consequent reflection of dysregulation involving the hematopoietic machinery in experimental mice. Swiss albino mice were experimentally exposed to 10 μg arsenic trioxide/g body weight through oral gavage and 5 μg arsenic trioxide/g body weight intraperitoneally for a period of 30 days. Altered hemogram values in peripheral blood reflected the impaired hematopoiesis which was further validated by the reduced BM cellularity along with the deviated BM cell morphology as observed by scanning electron microscopy post arsenic exposure. The stromal cells were unable to establish a healthy matrix and the sustainability of hematopoietic progenitors was drastically affected in arsenic-exposed mouse groups, as observed in in vitro explant culture. The inability of stromal cells to establish supportive matrix was also explained by the decreased adherent colony formation in treated animals. Furthermore, the flow cytometric characterization of CXCR4+ and Sca-1+ CD44+ receptor expressions confirmed the dysregulation in the hematopoietic microenvironment. Thus, considering the importance of microenvironment in the maintenance of HSPC, it can be concluded that arsenic toxicity causes microenvironmental damage, leading to niche derangement and impaired hematopoiesis.






Similar content being viewed by others
References
Bourdonnay E, Morzadec C, Sparfel L, Galibert MD, Jouneau S, Martin-Chouly C, Fardel O, Vernhet L (2009) Global effects of inorganic arsenic on gene expression profile in human macrophages. Mol Immunol 46(4):649–656
Bailey K, Xia Y, Ward WO, Knapp G, Mo J, Mumford JL, Owen RD, Thai SF (2009) Global gene expression profiling of hyperkeratotic skin lesions from inner Mongolians chronically exposed to arsenic. Toxicol Pathol 37(7):849–859
Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR (2008) Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect 116(4):524–531
Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Graziano JH, Gamble MV (2007) Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. Am J Clin Nutr 86(4):1179–1186
Gong Z, Lu X, Watt C, Wen B, He B, Mumford J, Ning Z, Xia Y, Le CX (2006) Speciation analysis of arsenic in groundwater from Inner Mongolia with an emphasis on acid-leachable particulate arsenic. Anal Chim Acta 555(1):181–187
Ezeh PC, Lauer FT, MacKenzie D, McClain S, Liu KJ, Hudson LG, Gandolfi AJ, Burchiel SW (2014) Arsenite selectively inhibits mouse bone marrow lymphoid progenitor cell development in vivo and in vitro and suppresses humoral immunity in vivo. PLoS One 9(4):e93920
Tchounwou PB, Patlolla AK, Centeno JA (2003) Carcinogenic and systemic health effects associated with arsenic exposure—a critical review. Toxicol Pathol 31(6):575–588
Vrotsos E, Martinez R, Pizzolato J, Martinez A, Sriganeshan V (2014) Arsenic exposure as a cause of persistent absolute eosinophilia. JMED Research. doi:10.5171/2014.230675
Flora SJS (2014) Toxic metals: health effects, and therapeutic measures. Journal of Biomedical and Therapeutic Sciences 1(1):48–64
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121(3):295–302
Wang HS, Sthiannopkao S, Chen ZJ, Man YB, Du J, Xing GH, Kim KW, Mohamed Yasin MS, Hashim JH, Wong MH (2013) Arsenic concentration in rice, fish, meat and vegetables in Cambodia: a preliminary risk assessment. Environ Geochem Health 35(6):745–755
Saha KC (1984) Melanokeratosis from arsenic contaminated tubewell water. Indian J Dermatol 29(4):37–46
Rebuzzini P, Cebral E, Fassina L, Alberto Redi C, Zuccotti M, Garagna S (2015) Arsenic trioxide alters the differentiation of mouse embryonic stem cell into cardiomyocytes. Sci Rep. doi:10.1038/srep14993
Moon K, Guallar E, Navas-Acien A (2012) Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 14(6):542–555
Mehta A, Kannan GM, Dube SN, Pant BP, Pant SC, Flora SJ (2002) Haematological, hepatic and renal alterations after repeated oral or intraperitoneal administration of monoisoamyl DMSA. I. Changes in male rats. J Appl Toxicol 22(6):359–369
Yu M, Xue J, Li Y, Zhang W, Ma D, Liu L, Zang Z (2013) Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol 87:1025–1035
Calderón J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V, Díaz-Barriga F (2001) Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res 85(2):69–76
Tsai SY, Chou HY, The HW, Chen CM, Chen CJ (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24(4–5):747–753
Liu S, Guo X, Wu B, Yu H, Zhang X, Li M (2014) Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci Rep. doi:10.1038/srep06894
Ratnaike RN (2003) Acute and chronic arsenic toxicity. Postgrad Med J 79(933):391–396
Gu QL, Li NL, Zhu ZG, Yin HR, Lin YZ (2000) A study on arsenic trioxide inducing in vitro apoptosis of gastric cancer cell lines. World J Gastroenterol 6(3):435–437
Yadav S, Shi Y, Wang F, Wang H (2010) Arsenite induces apoptosis in human mesenchymal stem cells by altering Bcl-2 family proteins and by activating intrinsic pathway. Toxicol Appl Pharmacol 244(3):263–272
Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JØ (2009) The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit 11(7):1322–1330
Waalkes MP, Liu J, Germolec DR, Trempus CS, Cannon RE, Tokar EJ, Tennant RW, Ward JM, Diwan BA (2008) Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Res 68(20):8278–8285
Islam T, Parvin S, Pervin M, Bari ASM, Khan MAHNA (2011) Effects of chronic arsenic toxicity on the haematology and histoarchitecture of female reproductive system of black Bengal black goat. Bangl J Vet Med 9(1):59–66
Dangleben NL, Skibola CF, Smith MT (2013) Arsenic immunotoxicity: a review. Environ Health. doi:10.1186/1476-069X-12-73
Patterson R, Vega L, Trouba K, Bortner C, Germolec D (2004) Arsenic induced alterations in the contact hypersensitivity response in BALB/c mice. Toxicol Appl Pharmacol 198(3):434–443
Lemarie A, Morzadec C, Bourdonnay E, Fardel O, Vernhet L (2006) Human macrophages constitute targets for immunotoxic inorganic arsenic. J Immunol 177(5):3019–3027
Bishayi B, Sengupta M (2003) Intracellular survival of Staphylococcus aureus due to alteration of cellular activity in arsenic and lead intoxicated mature Swiss albino mice. Toxicology 184(1):31–39
Harrison MT, McCoy KL (2001) Immunosuppression by arsenic: a comparison of cathepsin L inhibition and apoptosis. Int Immunopharmacol 1(4):647–656
Biswas D, Banerjee M, Sen G, Das JK, Banerjee A, Sau TJ, Pandit S, Giri AK, Biswas T (2008) Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol Appl Pharmacol 230(1):57–66
Taichman RS (2005) Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 105(7):2631–2639
Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2(4):313–319
Baldridge MT, King KY, Goodell MA (2011) Inflammatory signals regulate hematopoietic stem cells. Trends Immunol 32(2):57–65
Pereira JA, Das P, Chaklader M, Chatterjee S, Basak P, Chaudhuri S, Law S (2010) Effects of inorganic arsenic on bone marrow hematopoietic cells: an emphasis on apoptosis and Sca-1/c-Kit positive population. J Stem Cells 5(3):117–127
Lew YS, Brown SL, Griffin RJ, Song CW, Kim JH (1999) Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res 59(24):6033–6037
Srivastava R, Bhattacharya S, Chakraborty A, Chattopadhyay A (2015) Differential in vivo genotoxicity of arsenic trioxide in glutathione depleted mouse bone marrow cells: expressions of Nrf2/Keap1/P62. Toxicol Mech Methods 25(3):223–228
Vahidnia A, van der Voet GB, de Wolff FA (2007) Arsenic neurotoxicity—a review. Hum Exp Toxicol 26(10):823–832
Valorani MG, Germani A, Otto WR, Harper L, Biddle A, Khoo CP, Lin WR, Hawa MI, Tropel P, Patrizi MP, Pozzilli P, Alison MR (2010) Hypoxia increases Sca-1/CD44 co-expression in murine mesenchymal stem cells and enhances their adipogenic differentiation potential. Cell Tissue Res 341(1):111–120
Sugiyama T, Kohara H, Noda M, Nagasawa T (2006) Immunity, maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25(6):977–988
Acknowledgements
Authors are thankful to the Director of Calcutta School of Tropical Medicine for successful completion of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the institutional animal ethical committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pereira, J.A., Law, S. Microenvironmental Scenario of the Bone Marrow of Inorganic Arsenic-Exposed Experimental Mice. Biol Trace Elem Res 181, 304–313 (2018). https://doi.org/10.1007/s12011-017-1022-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1022-2