Abstract
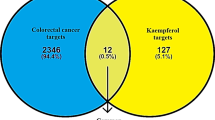
Our study aims to find the relevant mechanism of Mume Fructus in the treatment of triple-negative breast cancer (TNBC) by network pharmacology analysis and experimental validation. The effective compounds of Mume Fructus and TNBC-related target genes were imported into Cytoscape to construct a Mume Fructus-effective compounds-disease target network. The common targets of Mume Fructus and TNBC were determined by drawing Venn diagrams. Then, the intersection targets were transferred to the STRING database to construct a protein–protein interaction (PPI) network. To investigate the mechanism of Mume Fructus in treatment of TNBC, breast cancer cell (MDA-MB-231) was treated with Mume Fructus and/or transfected with small interference RNA-PKM2(siPKM2). CCK-8 assay, cell clonal formation assay, transwell, flow cytometry, qRT-PCR, and western blotting were performed. Eight effective compounds and 145 target genes were obtained, and the Mume Fructus- effective compounds-disease target network was constructed. Then through the analysis of the PPI network, we obtained 10 hub genes including JUN, MAPK1, RELA, AKT1, FOS, ESR1, IL6, MAPK8, RXRA, and MYC. KEGG enrichment analysis showed that JUN, MAPK1, RELA, FOS, ESR1, IL6, MAPK8, and RXRA were enriched in the Th17 cell differentiation signaling pathway. Loss of PKM2 and Mume Fructus both inhibited the malignant phenotype of MDA-MB-231 cells. And siPKM2 further aggravated the Mume Fructus inhibition of malignancy of breast cancer cells. Network pharmacology analysis suggests that Mume Fructus has multiple therapeutic targets for TNBC and may play a therapeutic role by modulating the immune microenvironment of breast cancer.












Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Change history
01 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12010-024-04969-5
Abbreviations
- TNBC:
-
Triple-negative breast cancer
- Id-1:
-
DNA and mRNA binding factor
- TILs:
-
Tumor-infiltrating lymphocytes
References
Crosby, D., Bhatia, S., Brindle, K. M., Coussens, L. M., Dive, C., Emberton, M., et al. (2022). Early detection of cancer. Science, 375(6586), eaay9040. https://doi.org/10.1126/science.aay9040
Garrido-Castro, A. C., Lin, N. U., & Polyak, K. (2019). Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discovery, 9(2), 176–198. https://doi.org/10.1158/2159-8290.CD-18-1177
Kudelova, E., Smolar, M., Holubekova, V., Hornakova, A., Dvorska, D., Lucansky, V. et al. (2022). Genetic heterogeneity, tumor microenvironment and immunotherapy in triple-negative breast cancer. International Journal of Molecular Sciences, 23(23). https://doi.org/10.3390/ijms232314937
Nielsen, T. J., Ring, B. Z., Seitz, R. S., Hout, D. R., & Schweitzer, B. L. (2021). A novel immuno-oncology algorithm measuring tumor microenvironment to predict response to immunotherapies. Heliyon, 7(3), e06438. https://doi.org/10.1016/j.heliyon.2021.e06438
Chabanon, R. M., Muirhead, G., Krastev, D. B., Adam, J., Morel, D., Garrido, M., et al. (2019). PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. The Journal of Clinical Investigation, 129(3), 1211–1228. https://doi.org/10.1172/JCI123319
Jungles, K. M., Holcomb, E. A., Pearson, A. N., Jungles, K. R., Bishop, C. R., Pierce, L. J., et al. (2022). Updates in combined approaches of radiotherapy and immune checkpoint inhibitors for the treatment of breast cancer. Frontiers in Oncology, 12, 1022542. https://doi.org/10.3389/fonc.2022.1022542
Geurts, V., & Kok, M. (2023). Immunotherapy for metastatic triple negative breast cancer: Current paradigm and future approaches. Current Treatment Options in Oncology, 24(6), 628–643. https://doi.org/10.1007/s11864-023-01069-0
Stanton, S. E., Adams, S., & Disis, M. L. (2016). Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncology, 2(10), 1354–1360. https://doi.org/10.1001/jamaoncol.2016.1061
Schreier, A., Zappasodi, R., Serganova, I., Brown, K. A., Demaria, S., & Andreopoulou, E. (2022). Facts and perspectives: Implications of tumor glycolysis on immunotherapy response in triple negative breast cancer. Frontiers in Oncology, 12, 1061789. https://doi.org/10.3389/fonc.2022.1061789
Liu, Z., Peng, Y., Ma, P., Fan, L., Zhao, L., Wang, M., et al. (2022). An integrated strategy for anti-inflammatory quality markers screening of traditional Chinese herbal medicine Mume Fructus based on phytochemical analysis and anti-colitis activity. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 99, 154002. https://doi.org/10.1016/j.phymed.2022.154002
Zhu, Y., Wei, S., Cao, X., Wang, S., Chang, Y., Ouyang, H., et al. (2022). Multi-component pharmacokinetic study of Prunus mume fructus extract after oral administration in rats using UPLC-MS/MS. Frontiers in Pharmacology, 13, 954692. https://doi.org/10.3389/fphar.2022.954692
Fan, H., Shen, L., Tang, Q., Xiong, P., Shou, Z., Liao, Y., et al. (2009). Effect of Wumeiwan on cytokines TNF-alpha, IL-6, IL-8, IL-10 and expression of NF-kappaBp65 in rats with ulcerative colitis. Journal of Huazhong University of Science and Technology Medical Sciences, 29(5), 650–4. https://doi.org/10.1007/s11596-009-0523-4
Wan, Y., Xu, L., Liu, Z., Yang, M., Jiang, X., Zhang, Q., et al. (2019). Utilising network pharmacology to explore the underlying mechanism of Wumei Pill in treating pancreatic neoplasms. BMC Complementary and Alternative Medicine, 19(1), 158. https://doi.org/10.1186/s12906-019-2580-y
Ma, N. X., Sun, W., Wu, J., Liu, S. L., Weng, L., Liu, Y. Q., et al. (2017). Compound Wumei powder inhibits the invasion and metastasis of gastric cancer via Cox-2/PGE2-PI3K/AKT/GSK3beta/beta-catenin signaling pathway. Evidence-Based Complementary and Alternative Medicine : eCAM, 2017, 3039450. https://doi.org/10.1155/2017/3039450
Feng, J., Zhao, J., Jia, W., Zhao, G., Peng, T. (2010) Effect of chemotherapy with modified Wu Mei Wan on advanced breast cancer. Shanxi of TCM, 26(7), 33–35. https://doi.org/10.3969/j.issn.1000-7156.2010.07.022
Liu, Y., An, T., Yu, H., Wan, D., Fan, Y., & Pei, X. (2021). Pharmacological mechanism of Wumeiwan in treating lung metastasis of breast cancer. Chinese Journal of Experimental Traditional Medical Formulae, 27(9), 193–201. https://doi.org/10.13422/j.cnki.syfjx.20210516
Xing, H., Zhang, L., Ma, J., Liu, Z., Song, C., & Liu, Y. (2018). Fructus mume extracts alleviate diarrhea in breast cancer patients receiving the combination therapy of lapatinib and capecitabine. Frontiers in Pharmacology, 9, 516. https://doi.org/10.3389/fphar.2018.00516
Zhang, W., Xue, K., Gao, Y., Huai, Y., Wang, W., Miao, Z., et al. (2019). Systems pharmacology dissection of action mechanisms of Dipsaci Radix for osteoporosis. Life Sciences, 235, 116820. https://doi.org/10.1016/j.lfs.2019.116820
Lu, D. X., Liu, F., Wu, H., Liu, H. X., Chen, B. Y., Yan, J., et al. (2022). Wumei pills attenuates 5-fluorouracil-induced intestinal mucositis through toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-kappaB pathway and microbiota regulation. World Journal of Gastroenterology, 28(32), 4574–4599. https://doi.org/10.3748/wjg.v28.i32.4574
Bu, S., Yuan, C., Cao, F., Xu, Q., Zhang, Y., Ju, R. et al. (2021). Concentrated extract of Prunus mume fruit exerts dual effects in 3T3-L1 adipocytes by inhibiting adipogenesis and inducing beiging/browning. Food & Nutrition Research, 65. https://doi.org/10.29219/fnr.v65.5492
Wang, S., Long, S., & Wu, W. (2018). Application of traditional Chinese medicines as personalized therapy in human cancers. The American Journal of Chinese Medicine, 46(5), 953–970. https://doi.org/10.1142/S0192415X18500507
Zhao, W., Li, B., Huang, J. (2015). Experimental study on the induction of apoptosis of pancreatic cancer sw1990 cells NOD-SCID mouse xenograft cells by Jiawei Wumei Pill. Chinese Journal of Emergency Medicine, 24(2), 194–196. https://doi.org/10.3969/j.issn.1004-745X.2015.02.003
Zhang Huizi, H. J. (2018). Experimental study on the inhibition and apoptosis of pancreatic cancer xenografts by Jiawei Wumei Pill combined with gemcitabine. Global Chinese Medicine, 11(7), 5.
Meehan, E. V., Wang, K. (2022). Interleukin-17 family cytokines in metabolic disorders and cancer. Genes 13(9). https://doi.org/10.3390/genes13091643
Loi, S., Michiels, S., Adams, S., Loibl, S., Budczies, J., Denkert, C., et al. (2021). The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: Clinical utility in an era of checkpoint inhibition. Annals of oncology: Official Journal of the European Society for Medical Oncology, 32(10), 1236–1244. https://doi.org/10.1016/j.annonc.2021.07.007
Mella, M., Kauppila, J. H., Karihtala, P., Lehenkari, P., Jukkola-Vuorinen, A., Soini, Y., et al. (2015). Tumor infiltrating CD8(+) T lymphocyte count is independent of tumor TLR9 status in treatment naive triple negative breast cancer and renal cell carcinoma. Oncoimmunology, 4(6), e1002726. https://doi.org/10.1080/2162402X.2014.1002726
Yeong, J., Thike, A. A., Lim, J. C., Lee, B., Li, H., Wong, S. C., et al. (2017). Higher densities of Foxp3(+) regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Research and Treatment, 163(1), 21–35. https://doi.org/10.1007/s10549-017-4161-4
Cimino-Mathews, A., Foote, J. B., & Emens, L. A. (2015). Immune targeting in breast cancer. Oncology, 29(5), 375–385.
Stanton, S. E., & Disis, M. L. (2016). Clinical significance of tumor-infiltrating lymphocytes in breast cancer. Journal for Immunotherapy of Cancer, 4, 59. https://doi.org/10.1186/s40425-016-0165-6
Paret, C., Simon, P., Vormbrock, K., Bender, C., Kolsch, A., Breitkreuz, A., et al. (2015). CXorf61 is a target for T cell based immunotherapy of triple-negative breast cancer. Oncotarget, 6(28), 25356–67. https://doi.org/10.18632/oncotarget.4516
Zhang, P., Yi, S., Li, X., Liu, R., Jiang, H., Huang, Z., et al. (2014). Preparation of triple-negative breast cancer vaccine through electrofusion with day-3 dendritic cells. PLoS One, 9(7), e102197. https://doi.org/10.1371/journal.pone.0102197
Ali, K., Soond, D. R., Pineiro, R., Hagemann, T., Pearce, W., Lim, E. L., et al. (2014). Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature, 510(7505), 407–411. https://doi.org/10.1038/nature13444
Mao, Y., Qu, Q., Chen, X., Huang, O., Wu, J., & Shen, K. (2016). The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PloS One, 11(4), e0152500. https://doi.org/10.1371/journal.pone.0152500
Asano, Y., Kashiwagi, S., Goto, W., Kurata, K., Noda, S., Takashima, T., et al. (2016). Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. The British Journal of Surgery, 103(7), 845–854. https://doi.org/10.1002/bjs.10127
Bi, J., Bi, F., Pan, X., & Yang, Q. (2021). Establishment of a novel glycolysis-related prognostic gene signature for ovarian cancer and its relationships with immune infiltration of the tumor microenvironment. Journal of Translational Medicine, 19(1), 382. https://doi.org/10.1186/s12967-021-03057-0
Soto-Heredero, G., Gomez De Las Heras, M. M., Gabande-Rodriguez, E., Oller, J., & Mittelbrunn, M. (2020). Glycolysis - a key player in the inflammatory response. The FEBS Journal, 287(16), 3350–3369. https://doi.org/10.1111/febs.15327
Freemerman, A. J., Johnson, A. R., Sacks, G. N., Milner, J. J., Kirk, E. L., Troester, M. A., et al. (2014). Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. The Journal of Biological Chemistry, 289(11), 7884–7896. https://doi.org/10.1074/jbc.M113.522037
Rossi, T., Zamponi, R., Chirico, M., Pisanu, M. E., Iorio, E., Torricelli, F., et al. (2023). BETi enhance ATGL expression and its lipase activity to exert their antitumoral effects in triple-negative breast cancer (TNBC) cells. Journal of Experimental & Clinical Cancer Research: CR, 42(1), 7. https://doi.org/10.1186/s13046-022-02571-3
Christofk, H. R., Vander Heiden, M. G., Harris, M. H., Ramanathan, A., Gerszten, R. E., Wei, R., et al. (2008). The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature, 452(7184), 230–233. https://doi.org/10.1038/nature06734
Dhanesha, N., Patel, R. B., Doddapattar, P., Ghatge, M., Flora, G. D., Jain, M., et al. (2022). PKM2 promotes neutrophil activation and cerebral thromboinflammation: Therapeutic implications for ischemic stroke. Blood, 139(8), 1234–1245. https://doi.org/10.1182/blood.2021012322
Doddapattar, P., Dev, R., Ghatge, M., Patel, R. B., Jain, M., Dhanesha, N., et al. (2022). Myeloid cell PKM2 deletion enhances efferocytosis and reduces atherosclerosis. Circulation Research, 130(9), 1289–1305. https://doi.org/10.1161/CIRCRESAHA.121.320704
Acknowledgements
We are thankful to Dr. Yuting Jiang for critically editing the current manuscript.
Funding
This work was supported by the Shandong Province Traditional Chinese Medicine Science and Technology Project, (2020M065); the Shandong Province School Health Association Project, (SDWS2022068) and the Shandong Province Traditional Chinese Medicine Science and Technology Project, (2017-263).
Author information
Authors and Affiliations
Contributions
The authors contributed equally.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, L., Qi, Y. & Jiang, Y. Pharmacological Mechanism of Mume Fructus in the Treatment of Triple-Negative Breast Cancer Based on Network Pharmacology. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04948-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04948-w