Abstract
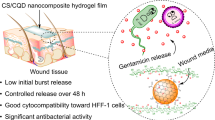
Today, the search for solutions to reduce wound infection and restore wound receptivity also reduces its side effects which are a difficult problem in medical science research. The greatest options for this purpose are hydrogel dressings since they are compatible with tissue and have an antibacterial effect on wound healing. Chronic wounds represent a significant burden on people and healthcare systems worldwide. Bacteria often enter such skin wounds, causing irritation and complicating the healing process. In addition, bacteria cause infection, which inhibits rejuvenation and the production of collagen. This study is aimed at developing novel chitosan (CS)—hydrolyzed starch nanocomposite (HS/Ch-NC) loaded with ciprofloxacin to enhance its skin retention and wound healing efficacy and anti-biofilm efficacy. Drug-loading on the (HS/Ch-NC) and encapsulation efficiency was 55.2% and 97.2%, respectively. The activity of HS-NC loaded with ciprofloxacin as anti-biofilm activity by 72% and 63% against Enterobacter aerogenes and Pseudomonas aeruginosa, respectively. The obtained (HS/Ch-NC) loaded with ciprofloxacin is a promising candidate for the development of improved bandage materials, as cell viability and proliferation was assessed using an SRB assay with half-maximal inhibitory concentrations (IC50) at 119.1 µg/ml. In vitro scratch wound healing assay revealed significant (p ≤ 0.05) acceleration in wound closure at 24 h enhanced by 56.04% 24-h and 100% 72-h post-exposure to (HS/Ch-NC) loaded ciprofloxacin, compared to the negative control. In vivo skin retention study revealed that (HS/Ch-NC)-loaded ciprofloxacin showed 3.65-fold higher retention, respectively, than ciprofloxacin. Thus, our study assumes that ciprofloxacin-loaded HS-NC is a potential delivery system for enhancing ciprofloxacin skin retention and wound healing activity.






Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Criollo-Mendoza, M. S., Contreras-Angulo, L. A., Leyva-López, N., Gutiérrez-Grijalva, E. P., Jiménez-Ortega, L. A., & Heredia, J. B. (2023). Wound healing properties of natural products: Mechanisms of action. Molecules, 28(2), 598.
Velnar, T., Bailey, T., & Smrkolj, V. (2009). The wound healing process: An overview of the cellular and molecular mechanisms. Journal of International Medical Research, 37(5), 1528–1542.
Kenawy, E., Abdel-Hay, F. I., MohyEldin, M., Tamer, T., & Ibrahim, E. (2015). Novel aminated chitosan-aromatic aldehydes Schiff bases: Synthesis, characterization and bio-evaluation. International Journal of Advanced Research, 3(2), 563–572.
Osama, S., Hussain, A. A., Mm, R., Shehabeldine, A. M., & Hasanin, M. S. (2022). Preliminary study for cellulolytic microorganism isolation from different resources, characterization and identification: Green convert of microcrystalline cellulose to nanofibers. Egyptian Journal of Chemistry, 65(131), 1265–1273.
Ahmed, S., & Ikram, S. (2016). Chitosan based scaffolds and their applications in wound healing. Achievements in the Life Sciences, 10(1), 27–37.
Shehabeldine, A. M., Hashem, A. H., Wassel, A. R., & Hasanin, M. (2022). Antimicrobial and antiviral activities of durable cotton fabrics treated with nanocomposite based on zinc oxide nanoparticles, acyclovir, nanochitosan, and clove oil. Applied Biochemistry and Biotechnology, 194(2), 783–800.
Dacrory, S., Hashem, A. H., & Kamel, S. (2022). Antimicrobial and antiviral activities with molecular docking study of chitosan/carrageenan@ clove oil beads. Biotechnology Journal, 17(2), 2100298.
Ibrahim, A. G., Elgammal, W. E., Hashem, A. H, Mohamed, A. E., Awad, M. A., & Hassan, S. M. (2023). Development of a chitosan derivative bearing the thiadiazole moiety and evaluation of its antifungal and larvicidal efficacy. Polymer Bulletin, 1–23. https://doi.org/10.1007/s00289-023-04765-x
Cui, C., Sun, S., Wu, S., Chen, S., Ma, J., & Zhou, F. (2021). Electrospun chitosan nanofibers for wound healing application. Engineered Regeneration, 2, 82–90.
He, Y., Zhao, W., Dong, Z., Ji, Y., Li, M., Hao, Y., Zhang, D., Yuan, C., Deng, J., & Zhao, P. (2021). A biodegradable antibacterial alginate/carboxymethyl chitosan/Kangfuxin sponges for promoting blood coagulation and full-thickness wound healing. International Journal of Biological Macromolecules, 167, 182–192.
Abdelghany, T. M., Al-Rajhi, A. M., Yahya, R., Bakri, M. M., Al Abboud, M. A., Yahya, R., Qanash, H., Bazaid, A. S., & Salem, S. S. (2023). Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass Conversion and Biorefinery, 13(1), 417–430.
Liu, H., Wang, C., Li, C., Qin, Y., Wang, Z., Yang, F., Li, Z., & Wang, J. (2018). A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC advances, 8(14), 7533–7549.
Mubeen, B., Ansar, A. N., Rasool, R., Ullah, I., Imam, S. S., Alshehri, S., Ghoneim, M. M., Alzarea, S. I., Nadeem, M. S., & Kazmi, I. (2021). Nanotechnology as a novel approach in combating microbes providing an alternative to antibiotics. Antibiotics, 10(12), 1473.
Salem, S. S., Hammad, E. N., Mohamed, A. A., & El-Dougdoug, W. (2022). A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Biointerface Research in Applied Chemistry, 13(1), 41.
Salem, S. S. (2023). A mini review on green nanotechnology and its development in biological effects. Archives of Microbiology, 205(4), 128.
Hashem, A. H., Al Abboud, M. A., Alawlaqi, M. M., Abdelghany, T. M., & Hasanin, M. (2022). Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity. Starch-Stärke, 74(1–2), 2100165.
Hasanin, M., Al Abboud, M. A., Alawlaqi, M. M., Abdelghany, T. M., & Hashem, A. H. (2022). Ecofriendly synthesis of biosynthesized copper nanoparticles with starch-based nanocomposite: Antimicrobial, antioxidant, and anticancer activities. Biological Trace Element Research, 200(5), 2099–2112. https://doi.org/10.1007/s12011-021-02812-0
Dacrory, S., Hashem, A. H., & Hasanin, M. (2021). Synthesis of cellulose based amino acid functionalized nano-biocomplex: Characterization, antifungal activity, molecular docking and hemocompatibility. Environmental Nanotechnology, Monitoring & Management, 15, 100453. https://doi.org/10.1016/j.enmm.2021.100453
Abu-Elghait, M., Hasanin, M., Hashem, A. H., & Salem, S. S. (2021). Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: Characterization, antibiofilm and biocompatibility. International Journal of Biological Macromolecules, 175, 294–303. https://doi.org/10.1016/j.ijbiomac.2021.02.040
Hashem, A. H., Hasanin, M., Kamel, S., & Dacrory, S. (2022). A new approach for antimicrobial and antiviral activities of biocompatible nanocomposite based on cellulose, amino acid and graphene oxide. Colloids and Surfaces B: Biointerfaces, 209, 112172. https://doi.org/10.1016/j.colsurfb.2021.112172
Lin, M., Liu, Y., Gao, J., Wang, D., Xia, D., Liang, C., Li, N., & Xu, R. (2022). Synergistic effect of co-delivering ciprofloxacin and tetracycline hydrochloride for promoted wound healing by utilizing coaxial PCL/gelatin nanofiber membrane. International Journal of Molecular Sciences, 23(3), 1895.
Elakraa, A. A., Salem, S. S., El-Sayyad, G. S., & Attia, M. S. (2022). Cefotaxime incorporated bimetallic silver-selenium nanoparticles: Promising antimicrobial synergism, antibiofilm activity, and bacterial membrane leakage reaction mechanism. RSC Advances, 12(41), 26603–26619.
Salem, S. S., Hashem, A. H., Sallam, A.-A.M., Doghish, A. S., Al-Askar, A. A., Arishi, A. A., & Shehabeldine, A. M. (2022). Synthesis of silver nanocomposite based on carboxymethyl cellulose: Antibacterial, antifungal and anticancer activities. Polymers, 14(16), 3352.
Shehabeldine, A., & Hasanin, M. (2019). Green synthesis of hydrolyzed starch–chitosan nano-composite as drug delivery system to gram negative bacteria. Environmental Nanotechnology, Monitoring & Management, 12, 100252.
Martins, L. G., & Mainardes, R. M. (2017). Application of a validated HPLC-PDA method for the determination of melatonin content and its release from poly (lactic acid) nanoparticles. Journal of pharmaceutical analysis, 7(6), 388–393.
Ohnishi, N., Yamamoto, E., Tomida, H., Hyodo, K., Ishihara, H., Kikuchi, H., Tahara, K., & Takeuchi, H. (2013). Rapid determination of the encapsulation efficiency of a liposome formulation using column-switching HPLC. International Journal of Pharmaceutics, 441(1–2), 67–74.
Kragh, K. N., Alhede, M., Kvich, L., & Bjarnsholt, T. (2019). Into the well—A close look at the complex structures of a microtiter biofilm and the crystal violet assay. Biofilm, 1, 100006.
Soliman, M. K., Salem, S. S., Abu-Elghait, M., & Azab, M. S. (2023). Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Applied Biochemistry and Biotechnology, 195(2), 1158–1183.
Shehabeldine, A. M., Amin, B. H., Hagras, F. A., Ramadan, A. A., Kamel, M. R., Ahmed, M. A., Atia, K. H., & Salem, S. S. (2023). Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: Time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Applied Biochemistry and Biotechnology, 195(1), 467–485.
Shehabeldine, A., El-Hamshary, H., Hasanin, M., El-Faham, A., & Al-Sahly, M. (2021). Enhancing the antifungal activity of griseofulvin by incorporation a green biopolymer-based nanocomposite. Polymers, 13(4), 542.
Shehabeldine, A. M., Ashour, R. M., Okba, M. M., & Saber, F. R. (2020). Callistemon citrinus bioactive metabolites as new inhibitors of methicillin-resistant Staphylococcus aureus biofilm formation. Journal of ethnopharmacology, 254, 112669.
Bai, J.-R., Wu, Y.-P., Elena, G., Zhong, K., & Gao, H. (2019). Insight into the effect of quinic acid on biofilm formed by Staphylococcus aureus. RSC advances, 9(7), 3938–3945.
Vajrabhaya, L.-o, & Korsuwannawong, S. (2018). Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. Journal of Analytical Science and Technology, 9(1), 1–6.
Hashem, A. H., Shehabeldine, A. M., Ali, O. M., & Salem, S. S. (2022). Synthesis of chitosan-based gold nanoparticles: Antimicrobial and wound-healing activities. Polymers, 14(11), 2293.
Doghish, A. S., Hashem, A. H., Shehabeldine, A. M., Sallam, A.-A.M., El-Sayyad, G. S., & Salem, S. S. (2022). Nanocomposite based on gold nanoparticles and carboxymethyl cellulose: Synthesis, characterization, antimicrobial, and anticancer activities. Journal of Drug Delivery Science and Technology, 77, 103874.
Farag, M. M., Ahmed, M. M., Abdallah, N. M., Swieszkowski, W., & Shehabeldine, A. M. (2020). The combined antibacterial and anticancer properties of nano Ce-containing Mg-phosphate ceramic. Life Sciences, 257, 117999.
Mohsen, A. M., Nagy, Y. I., Shehabeldine, A. M., & Okba, M. M. (2023). Thymol-loaded Eudragit RS30D cationic nanoparticles-based hydrogels for topical application in wounds: In vitro and in vivo evaluation. Pharmaceutics, 15(1), 19.
Cappiello, F., Casciaro, B., & Mangoni, M. L. (2018). A novel in vitro wound healing assay to evaluate cell migration. JoVE (Journal of Visualized Experiments), 133, e56825.
Lee, J.-H., Kim, H.-L., Lee, M. H., You, K. E., Kwon, B.-J., Seo, H. J., & Park, J.-C. (2012). Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine, 19(13), 1223–1227.
Shehabeldine, A. M., Salem, S. S., Ali, O. M., Abd-Elsalam, K. A., Elkady, F. M., & Hashem, A. H. (2022). Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. Journal of Fungi, 8(6), 612.
Abioye, A. O., Issah, S., & Kola-Mustapha, A. T. (2015). Ex vivo skin permeation and retention studies on chitosan–ibuprofen–gellan ternary nanogel prepared by in situ ionic gelation technique—A tool for controlled transdermal delivery of ibuprofen. International journal of pharmaceutics, 490(1–2), 112–130.
Assa, F., Jafarizadeh-Malmiri, H., Ajamein, H., Vaghari, H., Anarjan, N., Ahmadi, O., & Berenjian, A. (2017). Chitosan magnetic nanoparticles for drug delivery systems. Critical reviews in biotechnology, 37(4), 492–509.
Carrion, C. C., Nasrollahzadeh, M., Sajjadi, M., Jaleh, B., Soufi, G. J., & Iravani, S. (2021). Lignin, lipid, protein, hyaluronic acid, starch, cellulose, gum, pectin, alginate and chitosan-based nanomaterials for cancer nanotherapy: Challenges and opportunities. International Journal of Biological Macromolecules, 178, 193–228.
Badwan, A. A., Rashid, I., Al Omari, M. M., & Darras, F. H. (2015). Chitin and chitosan as direct compression excipients in pharmaceutical applications. Marine Drugs, 13(3), 1519–1547.
Schubert, J., & Chanana, M. (2019). Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Current Medicinal Chemistry, 25(35), 4556.
Punniyakotti, P., Panneerselvam, P., Perumal, D., Aruliah, R., & Angaiah, S. (2020). Anti-bacterial and anti-biofilm properties of green synthesized copper nanoparticles from Cardiospermum halicacabum leaf extract. Bioprocess and Biosystems Engineering, 43(9), 1649–1657.
Kushwaha, A., Rani, R., & Kumar, S. (2017). Mechanism of soil-metal-microbe interactions and their implication on microbial bioremediation and phytoremediation. In: P. Kumar, B. R. Gurjar & J. N. Govil (Eds.), Environmental science and engineering. Biodegradation and bioremediation (Vol. 8, 1st ed.). Studium Press LLC, New Delhi
Dhivya, S., Padma, V. V., & Santhini, E. (2015). Wound dressings–A review. Biomedicine, 5(4), 22.
Nagoba, B., Davane, M., Gandhi, R., Wadher, B., Suryawanshi, N., & Selkar, S. (2017). Treatment of skin and soft tissue infections caused by Pseudomonas aeruginosa—A review of our experiences with citric acid over the past 20 years. Wound Medicine, 19, 5–9.
Song, K., Hao, Y., Liu, Y., Cao, R., Zhang, X., He, S., Wen, J., Zheng, W., Wang, L., & Zhang, Y. (2023). Preparation of pectin-chitosan hydrogels based on bioadhesive-design micelle to prompt bacterial infection wound healing. Carbohydrate Polymers, 300, 120272.
Roy, D. C., Tomblyn, S., Burmeister, D. M., Wrice, N. L., Becerra, S. C., Burnett, L. R., Saul, J. M., & Christy, R. J. (2015). Ciprofloxacin-loaded keratin hydrogels prevent Pseudomonas aeruginosa infection and support healing in a porcine full-thickness excisional wound. Advances in Wound Care, 4(8), 457–468.
Huh, A. J., & Kwon, Y. J. (2011). “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. Journal of Controlled Release, 156(2), 128–145.
Kart, D., Reçber, T., Nemutlu, E., & Sagiroglu, M. (2021). Sub-inhibitory concentrations of ciprofloxacin alone and combinations with plant-derived compounds against P. aeruginosa biofilms and their effects on the metabolomic profile of P. aeruginosa biofilms. Antibiotics, 10(4), 414.
Acknowledgements
The authors express their sincere thanks to Faculty of science (Boys), Al-Azhar University, Cairo, Egypt, for providing the necessary research facilities; also, authors extend their appreciation to the researcher supporting project number (RSP2023R505), King Saud University, Riyadh, Saudi Arabia, for funding this work.
Funding
The authors extend their appreciation to the researcher supporting project number (RSP2023R505), King Saud University, Riyadh, Saudi Arabia, for funding publication fees.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shehabeldine, A.M., Al-Askar, A.A., AbdElgawad, H. et al. Wound Dressing Scaffold with High Anti-biofilm Performance Based on Ciprofloxacin-Loaded Chitosan-Hydrolyzed Starch Nanocomposite: In Vitro and In Vivo Study. Appl Biochem Biotechnol 195, 6421–6439 (2023). https://doi.org/10.1007/s12010-023-04665-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04665-w