Abstract
In this study, the second-life application of Saccharomyces cerevisiae obtained from brewery wastewater was evaluated in the biosorption of Se(IV) (Na2SeO3) sorbate in residue generated from a fine chemical industry. Biosorption experiments were carried out with different Se(IV) concentrations (A = 7.5 to 30.0 mg L-1 dissolved in deionized water or industrial effluent) and different biosorbent concentrations (B = 2.0 to 52.5 g L-1, dry mass). Inactive microbial biomass was evaluated in a wet and dehydrated state. The highest selenium removal efficiency (biosorption efficiency—R = 97.5%) was achieved with the same concentrations of sorbate in deionized water, using 24.0 g L-1 of wet cells. In contrast, the industrial effluent treatment showed lower biosorption efficiency (R = 83.3%) due to a large amount of other salts in the medium, mainly sulphur. Overall, the use of smaller amounts of biosorbent had a biosorption capacity of approximately five times greater than when 24.0 g L-1 in industrial effluent treatment was used. However, as reducing the concentration of the contaminant contained in the wastewater is the primary goal of this study, a more significant amount of biosorbent is recommended.
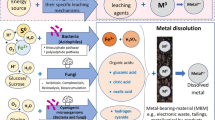
Graphical abstract






Similar content being viewed by others
Data Availability
Data from measurements were generated at the University of the Region of Joinville, Brazil. Derived data supporting the findings of this study are available from the corresponding author Elisabeth Wisbeck on request.
References
Buratto, A. P., Costa, R. D., & Ferreira, E. S. (2012). Application of fungal biomass of Pleurotus ostreatus in the process of biosorption of copper ions (II). Engenharia Sanitaria e Ambiental, 17, 413–420. https://doi.org/10.1590/S1413-41522012000400008
Schneider, I. A. H. (1995). Biossorção de Metais Pesados com a Biomassa de Macrófitos Aquáticos. In 157 f Tese Doutorado – Programa de Pós-Graduação em Engenharia Metalúrgica e dos Materiais. Universidade Federal do Rio Grande do Sul https://lume.ufrgs.br/handle/10183/96089
Santos, S., Ungureanu, G., Boaventura, R., & Botelho, C. (2015). Selenium contaminated Waters: an overview of analytical methods, treatment options and recent advances in sorption methods (Review). Science of the Total Environment, 521-522, 246–260. https://doi.org/10.1016/j.scitotenv.2015.03.107
Göksungur, Y., Üren, S., & Güvenç, U. (2005). Biosorption of cadmium and lead ions by ethanol treated waste baker´s yeast biomass. Bioresource Technology, 96, 103–109. https://doi.org/10.1016/j.biortech.2003.04.002
Park, D., Yun, Y. S., & Park, J. M. (2005). Use of dead fungal biomass for the detoxification of hexavalent chromium: screening and kinetics. Process Biochemistry, 40, 2559–2565. https://doi.org/10.1016/j.procbio.2004.12.002
Vasudevan, P., Padmavathy, V., & Dhingra, S. C. (2003). Kinetics of biosorption of cadmium on Baker’s yeast. Bioresource Technology, 89, 281–287. https://doi.org/10.1016/S0960-8524(03)00067-1
Di Caprio, F., Altimari, P., Uccelletti, D., & Pagnanelli, F. (2014). Mechanistic modeling of copper biosorption by wild type and engineered Saccharomyces cerevisiae biomasses. Chemical Engineering Journal, 244, 561–568. https://doi.org/10.1016/j.cej.2014.01.098
Vinopal, S., Ruml, T., & Kotrba, P. (2007). Biosorption of Cd2+ and Zn2+ by cell surface-engineered Saccharomyces cerevisiae. International Biodeterioration & Biodegradation, 60, 96–102. https://doi.org/10.1016/j.ibiod.2006.12.007
Marcellino, S., Attar, H., Lièvremont, D., Lett, M.-C., Barbier, F., & Lagarde, F. (2008). Heat-treated Saccharomyces cerevisiae for antimony speciation and antimony (III) preconcentration in water samples. Analytica Chimica Acta, 629, 73–83. https://doi.org/10.1016/j.aca.2008.09.031
Fadel, M., Hassanein, N. M., Elshafei, M. M., Mostafa, A. H., Ahmed, M. A., & Khater, H. M. (2017). Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. HBRC Journal., 13, 106–113. https://doi.org/10.1016/j.hbrcj.2014.12.006
Khakpour, H., Younesi, H., & Mohammadhosseini, M. (2014). Two-stage biosorption of selenium from aqueous solution using dried biomass of the baker’s yeast Saccharomyces cerevisiae. Journal of Environmental Chemical Engineering, 2, 532–542. https://doi.org/10.1016/j.jece.2013.10.010
Pankiewicz, U., Sujka, M., Kowalski, R., Mazurek, A., Stasiak, M. W., & Jamroz, J. (2017). Effect of pulsed electric fields (PEF) on accumulation of selenium and zinc ions in Saccharomyces cerevisiae cells. Food Chemistry, 221, 1361–1370. https://doi.org/10.1016/j.foodchem.2016.11.018
Wang, J., & Chen, C. (2006). Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnology Advances, 24, 427–451. https://doi.org/10.1016/j.biotechadv.2006.03.001
ASTM - American Society for Testing And Materials. (2019). E871–82: standard test method for moisture analysis of particulate wood fuels. West Conshokocken, PA.
ABNT - Associação Brasileira De Normas Técnicas. (1987). NBR 7217. Determinação da Composição Granulométrica http://licenciadorambiental.com.br/wp-content/uploads/2015/01/NBR-7.217-Determina%C3%A7%C3%A3o-da-composi%C3%A7%C3%A3o-granulom%C3%A9trica.pdf
Rice, E. W., Baird, R. B., Eaton, A. D., & Clesceri, L. S. (Eds.). (2012). Standard methods for the examination of water and wastewater (22nd ed.). American Public Health Association.
Rorabacher, D. B. (1991). Statistical treatment for rejection of deviant values: critical values of Dixon´s “Q” parameter and related subrange rations at the 95% confidence level. Analytical Chemistry, 63, 139–146. https://doi.org/10.1021/ac00002a010
Ghorbani, F., Younesi, H., Ghasempourim, S. M., Zinatizadeh, A. A., Amini, M., & Danesh, A. (2008). Application of response surface methodology for optimization of cádmium biosorption in an aqueous solution by Saccharomyces cerevisiae. Chemical Engineer Journal, 145, 267–275. https://doi.org/10.1016/j.cej.2008.04.028
Labuto, G., Trama, B., Gueller, G. C. S., Guarnieri, B. S., Silva, F. V., & Collazo, R. (2015). Metals uptake by live yeast and heat-modified yeast residue. Revista Ambiente & Água, 10, 510–519. https://doi.org/10.4136/ambi-agua.1577
El-Shafey, E. I. (2007). Sorption of Cd(II) and Se(IV) from aqueous solution using modified rice husk. Journal of Hazardous Materials, 147, 546–555. https://doi.org/10.1016/j.jhazmat.2007.01.051
Abdi, O., & Kazemi, M. (2015). A review study of biosorption of heavy metals and comparison between different biosorbent. Journal Material Environment Science, 6, 1386–1399 http://jmaterenvironsci.com/Document/vol6/vol6_N5/164-JMES-1454-2015-Abdi.pdf
Nascimento, J. M., Oliveira, J. D., Rizzo, A. C. L., & Leite, S. G. F. (2018). Biosorption Cu (II) by the yeast Saccharomyces cerevisiae. Biotechnology Reports, 20, 1–7. https://doi.org/10.1016/j.btre.2019.e00315
Malik, A. (2004). Metal Bioremediation through growing cells. Environment International, 30, 261–278. https://doi.org/10.1016/j.envint.2003.08.001
Shamim, S. (2018). Biosorption of heavy metals. In J. Derco & B. Vrana (Eds.), Biosorption (Vol. 2, pp. 21–49). IntechOpen. https://doi.org/10.5772/intechopen.72099
Atkinson, B. W., Bux, F., & Kasan, H. C. (1998). Considerations for application of biosorption technology to remediate metal contaminated industrial effluents. Water SA, 24, 129–135 https://www.wrc.org.za/wp-content/uploads/mdocs/WaterSA_1998_02_apr98_p129.pdf
Ahalya, N., Ramachandra, T. V., & Kanamadi, R. D. (2003). Biosorption of heavy metals. Research Journal of Chemistry and Environment, 7, 71–79 http://wgbis.ces.iisc.ernet.in/energy/water/paper/biosorption_heavymetals/Biosorption.pdf
Sekhar, K. C., Subramanian, S., Modak, J. M., & Natarajan, K. A. (1998). Removal of metal ions using an industrial biomass with reference to environmental control. International Journal of Mineral Processing, 53, 107–120. https://doi.org/10.1016/S0301-7516(97)00061-6
Hadiani, M. R., Darani, K. K., Rahimifard, N., & Younesi, H. (2018). Biosorption of low concentration levels of Lead (II) and Cadmium (II) from aqueous solution by Saccharomyces cerevisiae: Response surface methodology. Biocatalysis and Agricultural Biotechnology, 15, 25–34. https://doi.org/10.1016/j.bcab.2018.05.001
Twidwell, L., McCloskey, J., Miranda, P., & Gale, M. (1999). Technologies and potential technologies for removing selenium from process and mine wastewater. In Proceedings of the recycling, waste, treatment, and clean technology (REWAS) (pp. 1645–1656). San Sebastian.
Vijayaraghavan, K., & Balasubramanian, R. (2015). Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. Journal of Environmental Management, 160, 283–296. https://doi.org/10.1016/j.jenvman.2015.06.030
Santos, M. S., & Ribeiro, F. M. (2005). Cervejas e refrigerantes (p. 58). CETESB, São Paulo https://cetesb.sp.gov.br/consumosustentavel/wp-content/uploads/sites/20/2013/11/cervejas_refrigerantes.pdf
Acknowledgements
The authors thank the coordination for the Improvement of College Education Personnel – CAPES, Ministry of Education of Brazil, for the assistance with the scholarship, and to the INCASA S.A. Industry for the selenium analysis. The authors appreciate the support of the financiers and thank the Fund (FAP) from UNIVILLE.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Aieska Alves Gonçalves, Haira Gabriela Hackbarth, and Elisabeth Wisbeck. The first draft of the manuscript was written by Aieska Alves Gonçalves and Ozair Souza, and all authors commented on previous versions of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of Novelty
Currently, there is a need to develop effective methods capable of reducing or removing toxic components from residual waters, especially from activities that manipulate selenium compounds with different oxidation states. Biosorption can be a promising alternative for reduced selenium ions, using microbial biosorbents. Saccharomyces cerevisiae has received significant attention recently due to facile manipulation at the molecular level and safe classification as nonpathogenic. It has been verified that bakery commercial Saccharomyces cerevisiae demonstrates the possible application for the removal of selenium ions from aqueous solution in biosorption systems. However, in this study, the potential use of residual inactive Saccharomyces cerevisiae from the brewing industry was evaluated to remove Se(IV) ions present in chemical industry effluent. For instance, there is a reuse of problematic waste from the industry. The residual S. cerevisiae biomass becomes the biomass to carry out the selenium sorption. The biomass was employed in a wet state (without pre-treatment) and pre-treated in ethanol, followed by dehydration (dry biomass). The use of residual wet microbial biomass generated by the brewing industry showed potential use as a selenium biosorbent. This finding eliminates high investments for the treatment and discarded of this biomass generate. This result shows that this biomass can be used as a treatment for the residual water contaminated with Se. The importance of using biosorption concepts to treat effluents is motivated by costs lower than conventional treatments, especially those requiring large amounts of chemical products to treat large volumes of contaminated wastewater.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonçalves, A.A., Hackbarth, H.G., Wisbeck, E. et al. Evaluation of Residual Yeast from Brewery Industry for Inactive Biosorption of Selenium from Industrial Wastewater: a Case Study. Appl Biochem Biotechnol 196, 314–331 (2024). https://doi.org/10.1007/s12010-023-04549-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04549-z