Abstract
The global transition to a circular economy calls for research and development on technologies facilitating sustainable resource recovery from wastes and by-products. Metal-bearing materials, including electronic wastes, tailings, and metallurgical by-products, are increasingly viewed as valuable resources, with some possessing comparable or superior quality to natural ores. Bioleaching, an eco-friendly and cost-effective alternative to conventional hydrometallurgical and pyrometallurgical methods, uses microorganisms and their metabolites to extract metals from unwanted metal-bearing materials. The performance of bioleaching is influenced by pH, solid concentration, energy source, agitation rate, irrigation rate, aeration rate, and inoculum concentration. Optimizing these parameters improves yields and encourages the wider application of bioleaching. Here, we review the microbial diversity and specific mechanisms of bioleaching for metal recovery. We describe the current operations and approaches of bioleaching at various scales and summarise the influence of a broad range of operational parameters. Finally, we address the primary challenges in scaling up bioleaching applications and propose an optimisation strategy for future bioleaching research.
Similar content being viewed by others
Introduction
Metals, metalloids, and rare earth elements, collectively termed ‘metals’, are in high demand as they are essential to develop modern cities and technology products (Lee et al. 2022a). Catering the ever-increasing metal demand is become arduous due to the decrease in ore quality (IEA 2021). Rare earth elements and lithium (Li), cobalt (Co), antimony (Sb), copper (Cu) and indium (In) are labelled as critical metals due to their supply risk and economic importance (Gutiérrez-Gutiérrez et al. 2015; Muddanna and Baral 2021). Although metals are recyclable, the end-of-life recovery rate is low, < 50% for most of critical and base metals and < 1% for Li and rare earth elements (Muddanna and Baral 2021; IEA 2021). This is because metals are often trapped in the complex metal-bearing materials, e.g., metallurgical by-products, catalysts, Li-ion batteries, and electric-electronic equipment (Binnemans et al. 2020; Işıldar et al. 2019; Moazzam et al. 2021). Last decade, the end-of-life metal-bearing materials, can be also called as metal bearing wastes, has been widely accepted as secondary resource of critical raw materials due to metal content of metal-bearing wastes is comparable with natural ores (Işıldar et al. 2016; Lee et al. 2022a; Sarker et al. 2022). For example, electronic wastes contain up to 26 times higher Cu and 50 times higher Au content compared to ores/concentrates (Akcil et al. 2015). Another example is that some tailings can have higher Co content (0.02–1.38%) than a natural ore (0.05–0.3%) (Gutiérrez-Gutiérrez et al. 2015; Sarker et al. 2022). Similarly, the quality of some basic oxygen furnace dust, a metallurgical by-product, is close or better than virgin iron ores (Ma 2016).
Metal-bearing wastes, such as electronic waste, tailings, slag, and dusts, are typically stockpiled or landfilled due to the lack of efficient and sustainable recovery routes for the metals they contain (Binnemans et al. 2020). For instance, the UK alone has over 190 million tonnes of iron and steel waste stockpiled in current and former metallurgy sites (Riley et al. 2020). This underutilisation of metal-bearing material resources leads to economic losses and creates environmental challenges. To address this issue, enhanced landfill mining and urban mining have been proposed to recover secondary resources from stockpiled metal bearing wastes and integrate them into the circular economy (Arya and Kumar 2020a; Jones et al. 2013; Lee et al. 2022b). Enhanced landfill mining and urban mining align with the goal of the bioeconomy to create more sustainable value chains and improve productivity and quality of products in economic sectors. Moreover, recovering metals from metal-bearing wastes not only provides economic benefits and waste reduction but also allows for land reuse and treatment of heavy metal contamination. (BIT II 2019; Gao et al. 2021; Potysz et al. 2018; Sur et al. 2018). However, metal-bearing wastes recovery remains a challenge due to the complex and refractory structure of the materials. Therefore, there is a need for the development of efficient, sustainable, and low-cost recovery routes for metals from metal-bearing wastes (Binnemans et al. 2020; Potysz et al. 2021Yaashika et al. 2022).
The two main methods, pyrometallurgy and hydrometallurgy, are used to extract metals from primary (ore) and secondary sources e.g., tailings, e-waste, fly ash (Keshavarz et al. 2021; Potysz et al. 2018; Shahbaz 2022). Hydrometallurgy is the process used to extract metals from solid matrices, which is achieved by recovering and dissolving the metals as salt in successive aqueous solutions-based steps, including leaching, purification, and recovery of the targeted metal by selective precipitation or electrowinning (Dutta et al. 2023). Hydrometallurgy also involves using water for the extraction of metals, but the extraction and purification are heat-based processes (from ambient to around 300 °C). Extraction above 100 °C is carried out under pressure to prevent boiling (Whitworth et al. 2022). In pyrometallurgy, there are typically three steps including: roasting, smelting in high temperatures (250–1000 °C) and refining processes involving different kinds of furnaces and electrolytic processes (Arya and Kumar 2020a; Mishra et al. 2022). The disadvantages of pyrometallurgy are the loss of metals during the process for example the loss of lithium form Li-ion batteries, and the production of hazardous gases (Roy et al. 2021a, 2021b). With pyrometallurgy, a vast amount of energy and high capital cost is required, compared to hydrometallurgy processes (Arya and Kumar 2020a).
Bioleaching, which is also known as biomining, emerges as a promising eco-friendly and cost-effective biohydrometallurgical to traditional mining and metal recovery methods (Asghari et al. 2013; Gavrilescu 2022; Moazzam et al. 2021). Bioleaching is mediated by wide range of microorganisms and their metabolites (Fig. 1 and Table 1) (Gavrilescu 2022). The efficiency of bioleaching relies on operational parameters such as solid–liquid ratio, pH, energy source and concentration, and aeration rate, which are crucial for the recovery of metals from various solid matrices (see Supplementary Material Tables S1–S7). The process can be performed on different scales depending on the particle size of the metal-bearing material, with small to intermediate scale operations conducted in shake flasks, column reactors, and stirred bioreactors (Amiri et al. 2011a; Gomes et al. 2018; see Supplementary Material Tables S8–S11). Commercial scale operations are typically conducted ex-situ using vat, agitated tank reactors, heap, and dump methods (Natarajan 2018; Zanbak 2012). Although bioleaching has been industrialised for low-grade copper and refractory gold ores using acidophiles, it has not been widely adopted for other metal-bearing materials (Srichandan et al. 2019).
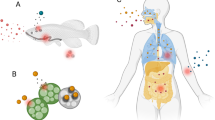
The bioleaching process. At the top, the figure shows inputs for bioleaching process which are energy sources depending on microorganisms' type and microorganisms with their specific leaching mechanism. Microorganisms produce biogenic leaching agents which can be ferric iron, sulphuric acid, organic acids, and hydrogen cyanide. Then, biogenic leaching agents react with metal-bearing materials to dissolve metals. M0 represents a metal in elemental state, Mn+ represents a metal in ionic form is metal in elemental state
This review provides a concise overview on the microbial diversity and associated bioleaching mechanisms used for solubilising metals. It then provides insights into their use through different bioleaching application strategies for various metal-bearing-materials from lab-scale to pilot scale applications and summarises the key parameters influencing bioleaching process. The review further sheds light on the remaining challenges for scale up alternatives and proposes an optimisation framework for future bioleaching research.
Microbial diversity and biological mechanisms
Based on their energy source preferences, microorganisms used for bioleaching are classified as chemolithotrophs, which oxidise inorganic compounds such as iron and sulphur to grow, and chemoorganotrophs, which oxidise reduced organic compounds (Srichandan et al. 2019, 2020). Chemolithotrophs are also commonly called acidophiles as they thrive under low pH values. Depending on their optimal growth temperatures, they can be further categorised as mesophiles, moderate thermophiles, and thermophiles (Natarajan 2018). Chemolithotrophs can be classified as chemolithotrophic autotrophs, which utilises inorganic matter, and chemolithotrophic heterotrophs, which utilise organic carbon as an energy source. Acidophiles have been the most used group of microorganisms for bioleaching application, followed by fungi and cyanogenic microorganisms (see Supplementary Material for details).
Acidophilic bioleaching
Acidophilic microorganisms, mainly bacteria, are often used to extract metals from sulphidic ores e.g., pyrite. Most common bacteria are Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans (Table 1) (Mikoda et al. 2019; Yi et al. 2021). These microorganisms oxidize ferrous iron (Fe+2) and/or sulphur compounds to produce ferric iron (Fe+3) and sulphuric acid (H2SO4) which are effective oxidizing agents for metal solubilisation. For instance, A. ferrooxidans oxidizes ferrous iron and sulphur compounds, A. thiooxidans only oxidizes elemental sulphur and Leptospirillum ferrooxidans and Leptospirillum ferriphilum only use ferrous iron as energy source.
Acidophiles typically use two pathways to solubilise metals from sulphidic ores: the thiosulphate and the polysulphide pathways. Ores such as pyrite, tungstenite, and molybdenite follow the thiosulphate pathway (Mishra et al. 2005; Srichandan et al. 2020). These ores do not soluble in acid; however, they are solubilised by Fe+3. Thiosulfate pathway for pyrite ore oxidation with A. ferrooxidans can be expressed by the following Eqs. (1–3)
On the other hand, ores such as chalcopyrite, arsenopyrite, sphalerite and galena are solubilised by collaboration of Fe+3 and H2SO4 or by H2SO4 only (Srichandan et al. 2020). Polysulfide oxidation pathway with A. ferrooxidans can be expressed by the following Eqs. (4–6):
Besides sulphidic form, metals are found as oxide and organically bound forms also present as hydroxides, carbonates, and silicates. Almost all acidophiles can manage to solubilise metals from these metallic forms at suitable conditions (Srichandan et al. 2020). Spent catalyst, fly ash and metallurgical slags can be given as examples of oxide form while sewage sludge is an organically bound metal form (Srichandan et al. 2020; Kremser et al. 2021). Thus, bioleaching has been one of the promising biotechnology applications used for recovering metal from wastes from these metal-bearing wastes (Ilyas and Lee 2014; Villares et al. 2016).
A. ferrooxidans has the unique ability to oxidize ferrous iron and elemental sulphur to ferric iron and sulphuric acid, respectively (Asghari et al. 2013; Wang et al. 2016). Benzal et al. (2020) investigated copper bioleaching from printed circuit boards from waste mobile phones by using A. ferrooxidans. 95–100% copper recovery was obtained in only 48 h using two-step bioleaching process. Chen et al., (2015) examined the feasibility of copper recovery from waste printed circuit boards by using A. ferrooxidans. 95% copper recovery was achieved after 28 days. Both Benzal et al., (2020) and Chen et al., (2015) stated that copper bioleaching mechanism can be expressed by the following Eqs. (7–9):
Gholami et al. (2011) investigated aluminium (Al), Co, molybdenum (Mo), and nickel (Ni) recovery from a spent processing catalyst by using A. ferrooxidans. 63% Al, 96% Co, 84% Mo and 99% Ni dissolution were achieved after 30 days. Wang et al. (2009a) studied Copper, Lead and Zinc mobilization from printed wire boards by using A. ferrooxidans. 99% Cu recovery was achieved after 9 days, and more than 88.9% Zn and Pb become soluble after 5 days of leaching time. They indicated Zn and Pb solubilisation mechanisms by Fe+3 as following Eqs. (10–11):
Fungal bioleaching
Aspergillus niger and Penicillium simplicissimum are the most common types of fungi found in bioleaching processes (Table 1). They produce various organic acids by using organic carbons such as glucose and sucrose as an energy source. The production of organic acids allows to dissolve metals from its ore or waste material. Among the organic acids produced, gluconic, citric, and oxalic acids have been shown as the most potent acids for bioleaching (Asghari et al. 2013; Srichandan et al. 2019). Fungi convert glucose or sucrose to organic acids through several enzymatic reactions in cytosol and mitochondrion which are membrane-bound cellular compartments. By passing through cytoplasmic membrane to cytosol, glucose is converted into pyruvate via the glycolysis pathway. One of the produced two pyruvate molecules is decarboxylated to acetyl-CoA by mitochondrion via malate (Trivedi et al. 2022). The other pyruvate carboxylates to oxaloacetic acid in the cytosol. Oxaloacetic acid is transferred into the mitochondrion and produce citric acid by condensed with acetyl-CoA. In cytosol, oxaloacetic acid is converted into oxalic acid and acetic acid by oxaloacetase (Srichandan et al. 2019; Trivedi et al. 2022).
Dissolution of metals by using fungi generated organic acids follows acidolysis, complexolysis, redoxolysis and bioaccumulation pathways (Asghari et al. 2013). In the acidolysis process, fungi-generated organic acids provide protons, and these protons react with metal on the ore or waste surface. Metal ions generated from acidolysis step become stabilize in the complexolysis process. Complexation occurs between metal ion(s) and organic acid(s). Generalised acidolysis and complexolysis reactions for dissolution of Ni ion can be expressed with Eqs. 12 and 13 and as follows (Asghari et al. 2013):
In redoxolysis step, microorganisms dissolve metals through oxidation–reduction processes. Asgrahi et al. (2013) stated that redox processes have a minor role in fungal leaching; however, oxidation–reduction processes have a significant role in bioleaching employed chemolithoautotrophic microorganisms. Redoxolysis reaction for dissolution of Mn ion can be expressed as following equation (Asghari et al. 2013):
Bioaccumulation mechanisms can be explained as actively transported metals through cell membrane accumulated into the cells and metal solubilisation continuously occur by disturbing equilibrium (Asghari et al. 2013; Srichandan et al. 2019).
Cyanogenic bioleaching
Cyanogenic microorganisms use organic carbon as energy source and mostly belong to Proteobacteria while there are few fungal species (Table 1). Their ability to produce cyanide, which is a good leaching agent, from glycine (\({\mathrm{NH}}_{2}{\mathrm{CH}}_{2}\mathrm{COOH}\)) through several metabolic activities makes them promising microorganisms for precious metal recovery from ore and wastes (Arab et al. 2020; Brandl et al. 2008; Faramarzi et al. 2020; Liu et al. 2016).
Studies on Chromobacterium violaceum, Pseudomonas putida and Pseudomonas fluorescens showed that bioleaching mechanism is based on the neutral process in which produced cyanide (lixivant) derived from bio-generated hydrogen cyanide dissolves metals by forming a metal complex (Asghari et al. 2013; Faramarzi et al. 2020). Production of hydrogen cyanide from glycine is carried out by HCN synthase enzyme which is encoded by hcnABC operon. Hydrogen cyanide synthesis reaction equation can be expressed by following equation (Srichandan et al. 2019):
According to Liu et al., (2016), bioleaching of gold by cyanogenic microorganisms typically involves an indirect process where biologically generated HCN forms soluble metallic complexes, such as [Cu(CN2)]−, [Ag(CN2)]−, [Au(CN2)]− (Pourhossein et al. 2021). The equations proposed in this process are represented by Eqs. (16–18) as follows:
Bioleaching mechanisms, approaches, and processes parameters
Bioleaching mechanisms
There are two microbial leaching mechanisms that can be used to dissolve metals from ore or waste materials, which are classified based on their contact status: direct (Eqs. 19 and 20) and indirect (Eqs. 21–23) mechanisms. In the direct mechanism, microorganisms attach onto the ore/waste material surface via extracellular polymeric substances (Op de Beeck et al. 2021; Costa et al. 2018; Moazzam et al. 2021) and simultaneously dissolve metals with several reactions occurring in the region of the extracellular polymeric substances (He et al. 2014; Wang et al. 2018). In the indirect mechanism, the planktonic microorganism’s mediate dissolution process by producing leaching agents such as Fe+3, H2SO4, organic acids, HCN (Table 1) (Natarajan 2018). These biogenic leaching agents dissolve metals in the ore or waste material without requiring a microbial attachment. As an example, dissolution of pyrite by A. ferrooxidans is shown in the Eqs. (19–23) (Chen and Lin 2010; Sand et al. 2001):
Direct mechanism
Indirect mechanism
Direct and indirect mechanisms are also called as contact and non-contact leaching, respectively. A third term, “cooperative leaching” is used to describe the combination of both contact and noncontact mechanism (Natarajan 2018). In cooperative leaching, the microorganisms attach to the surface of metal-bearing-material (Eqs. 19–20) and the free cells in the solution simultaneously mediate dissolution (Eqs. 21–23). The indirect/direct mechanism phenomena have led to development of different bioleaching engineering approaches in practice which are one-step, two-step, and spent-medium step bioleaching.
Bioleaching operations and approaches
Scale of bioleaching applications
Methods in bioleaching are mainly classified as agitation leaching for fine particles and percolation leaching process for coarse particles (Fig. 2; Natarajan 2018). At laboratory scale, most of the studies have been done in shake flask to reduce process complications and to obtain valuable information about the bioleaching process (Srichandan et al. 2020; Table S8). Process parameters such as pH, energy source concentration, inoculum and solid concentration and agitation speed can be optimised in shake flask (Amiri et al. 2011a; Bayat et al. 2008). However, it is not sufficient to understand leaching kinetics to design commercial scale reactors.
Industrial scale applications using percolation methods are mostly on-site ex-situ methods which are heap, dump, vat reactor and the in-situ leaching method (Fig. 2). Stirring method is used in commercial scale agitated tank reactor (ex-situ) (Maluckov, 2017; Zanbak, 2012; Table 2). Heap and dump have a similar construction, however; the difference is that to build heap metal-bearing-materials are subjected to size reduction, < 25 mm, and placed on leach pads (Natarajan 2018; Table 3). Microorganisms are maintained in a separate reservoir and irrigated on the top of the metal-bearing-materials. In-situ bioleaching is performed underground using natural porosity of rocks or creating porosity by blasting (Abhilash and Pandey, 2013; Vargas et al. 2020). Vat bioleaching is used as cost effective method to bioleach fine materials, 1–10 mm, in submerged condition, yet it is not prominent in commercial bioleaching operations (Natarajan 2018; Watling 2014). On commercial scale agitated tank reactors, the microorganisms and the metal-bearing-materials are maintained in the same place. Also, the complex process parameters are better controlled and observed in stirred condition for instance microbial growth kinetics, gas–liquid-solid mass transfer and temperature (Gericke et al. 2009; Natarajan 2018). However, compared to heap, it has drawback because it is only efficient up to 20% solid concentration (Natarajan 2018).
From shake flask scale to production at industrial scale, column reactors and stirred bioreactors are used to perform intermediary scale operation. Lab scale stirred bioreactor is used to simulate commercial scale agitated tank bioleaching (often > 1000 m3) (Natarajan 2018). Column bioleaching provides flexibility to optimise heap and dump specific parameters for example irrigation rate of the leachate. Microorganisms are mainly maintained in a separate reservoir. Column reactor can be operated in batch or recycling mode based on which one is desired to select to operate heap bioleaching (Srichandan et al. 2020). It can be operated both in fluidized bed, submerged, or drain mode, free flow, according to particle size (Natarajan 2018; Pathak et al. 2019). Although aerated column is a good design to simulate a heap construction, there are studies have been tested column without aeration. (Yang et al. 2013; Abhilash et al. 2010).
Bioleaching approaches
There are three approaches to operate microorganisms for bioleaching including: one-step (Fig. 3a, b c), two-step (Fig. 3d, e f) and spent-medium step (Fig. 3g, h, i) bioleaching approaches (Moazzam et al. 2021). Each approach can yield different leaching efficiency mainly because of the metal toxicity and the difference of the associated bioleaching mechanism which are direct, indirect, or cooperative leaching mechanisms (Amiri et al. 2011b). In shake flask or stirred bioreactor, one-step bioleaching approach is applied by adding cells and the metal-bearing-material into a fresh growth medium at the same time (Fig. 3b). Thus, microbial growth and metal dissolution begin simultaneously. In this approach, microorganisms are however often adversely affected by metal toxicity as well as alkaline nature of metal-bearing wastes before they have time to produce sufficient leaching agents resulting in insufficient metal dissolution (Srichandan et al. 2020; Kremser et al. 2022). In the two-step bioleaching (Fig. 3e), first, microorganisms are added into a fresh growth medium. Then, cell cultures are incubated for 2–7 days or even more, depending on the microbes used, in the absence of metal-bearing-material to allow microorganism to grow and produce leaching agents. Incubation time is 2 days or more for iron-oxidizing bacteria until the oxidation–reduction potential is ≥ 600 mV or until reduction is observed in pH in case of sulfur-oxidizing bacteria. Then the metal-bearing-material is added into the culture which still contains cells. In the spent-medium step (Fig. 3h) bioleaching approach, can be also called cell-free bioleaching, cells are initially cultured until reaching the stationary phase of metabolites production. This follows removing cells to create cell-free, supernatant, medium. Then, metal-bearing-material is added in this cell-free medium (Moazzam et al. 2021). Both two-step and spent-medium bioleaching approaches usually provide better dissolution yield than one-step bioleaching (Amiri et al. 2011b). Spent-medium bioleaching can provide more flexible industrial application due to metal-bearing-material being not contaminated by microorganisms (Moazzam et al. 2021).
Bioleaching approaches used for metal solubilisation a mechanism of the one-step bioleaching approach; in this approach, both microorganisms and metal-bearing material are added to the medium at the same time, and the bioleaching process is carried out during the microorganisms culturing b one-step approach in shake flask, c one-step approach in column, d mechanism of the two-step bioleaching approach; in this approach, first, the microorganisms are cultivated in the absence of solid waste, then after microorganisms are reached the log phase, metal-bearing material is added into the culture to start the bioleaching, e two-step approach in shake flask, f two-step approach in column, g mechanism of the spent-medium step bioleaching approach; in this approach, the microorganisms are cultivated until stationary phase to produce maximum biogenic leaching agent. Then after microorganism are removed from lixiviant metal-bearing material is added into the cell free medium to start bioleaching, h spent-medium step approach in shake flask, i spent-medium step approach in column (Moazzam et al. 2021). EPS is the abbreviation of extracellular polymeric substances, HCN is hydrogen cyanide, M0 represents a metal in elemental state, Mn+ represents a metal in ionic form
In bioleaching using the percolation method, the cells are typically kept separate from the metal-bearing material and maintained in a reservoir or external bioreactor (Fig. 3c, f, i). The success of this approach depends on the location of inoculation, either in the column or reservoir. In laboratory-scale column operations, inoculating cells at the top of the column creates a one-step bioleaching process (Fig. 3c), but this may result in lower yield (< 20%) or longer operation time (> 100 days) due to metal toxicity (Ilyas et al. 2010b). Alternatively, a two-step approach involves first inoculating cells into a reservoir and allowing them to reach the log phase before feeding them into the column (Fig. 3f; Mousavi et al. 2006). Another option is the spent-medium step (Fig. 3i), where cells are first cultivated until the stationary phase of metabolite production and then removed to create cell-free medium that is fed into the column.
Conditions and parameters controlling the bioleaching efficiency
Bioleaching efficiency is influenced by a wide range of parameters and conditions including pH, particle size, pre-treatment, solid concentration, inoculum concentration, energy source and concentration, agitation/irrigation rate, aeration rate, temperature, and catalysts.
Particle size
Particle size is a significant parameter for bioleaching in terms of both affecting leaching performance and guiding industrial application method (Natarajan 2018). When the particle size decreases the available surface area increases. The rise in the available surface area provides high bioleaching yield and increase microorganism interaction with ore/waste (Wang et al. 2018). Particles in bioleaching can be classified as either fine or coarse, with fine particles being less than 1 mm in size, and coarse particles ranging from 1 mm to over 25 mm depending on the type of leaching method used as follows: 1–10 mm (vat leaching); 10–25 mm (heap leaching); 25 mm < (dump leaching) (Natarajan 2018). Fine particles have a higher potential for clogging, but this can be prevented by using acid agglomeration techniques (Srichandan et al. 2020). Additionally, fine particles can have a stronger buffering effect, while larger particle sizes can reduce buffering but can also lead to lower dissolution rates and longer operation periods due to limited mass transfer (Srichandan et al. 2020).
Pre-treatment
As a pre-treatment, acid washing, sterilisation, water washing and thermal treatment, are applied to increase the bioleaching performance in some cases (Chu et al. 2022; Wang et al. 2009b). When acidophiles are employed, acid washing is often performed for material which is alkaline in nature. To favour the microorganism pH stabilisation, ≤ pH 2, is carried out to by using dilute sulphuric acid (Chen et al. 2015; Ilyas et al. 2010b). Sterilisation by autoclaving, for 15–30 min at 121–135 °C, is often applied in lab scale bioleaching to remove indigenous microorganisms from metal-bearing-material to interpret the employed microorganisms’ actual performance (Tezyapar Kara et al. 2022; Gomes et al. 2018; Wang et al. 2016). Water-washing are applied for removing water soluble inorganic salts from metal-bearing-materials. It has been found increased markedly the dissolution of Cd, Mn, and Zn from 76%, 45%, 53% to 96%, 91%, 68%, respectively, from municipal solid waste incinerator fly ash by A. Niger (Wang et al. 2009b).
Thermal pre-treatment is applied in some cases to remove organic matter from metal-bearing-material due to iron and sulphur-oxidising acidophiles are known to be sensitive to organic compounds (Torma and Itzkovitch 1976). For example, Cu dissolution markedly increased from 47 to 100% after calcination of waste printed circuit boards in muffled furnace at 600 °C for 60 min (Chu et al. 2022). Similarly, Mo and Co recovery from spent refinery catalysts increased from 18 to 100% and 79% to 94%, respectively, after thermal treatment at 400 °C for 1–2 h, in acidophilic bioleaching (Qian et al. 2020). On the other hand, more than 60% of Co, Ni, Cu, Zn, Fe release from polymetallic ore and 80–90% of U, Cu, Ni, Mn, Mo, Y and Zn dissolution from polymetallic black shale, with up to 10% organic matter content, have been reported by acidophiles (Bhatti 2015; Watling et al. 2014). So, bioleaching can be performed for material contains up to 10% of organic content efficiently (> 60% metal dissolution). In addition, Chu et al. (2022) stated that while thermal pre-treatment increased Cu dissolution from 47 to 100%, it decreased Ni dissolution from 100 to 35% due to the formation of acid insoluble NiO. Therefore, thermal pre-treatment can be considered for selective metal extraction.
pH and oxidation–reduction potential
Oxidation–reduction potential and pH directly affect microbial activity, metal solubility, and generation of precipitation layers such as jarosite (Chen et al. 2015; Halinen et al. 2009; Zare Tavakoli et al. 2017c; Zhao et al. 2019). Ores and solid wastes contain acid consuming minerals such as sodium (Na), potassium (K), calcium (Ca) and magnesium (Mg). When acidophilic microorganisms are employed, these high reactive minerals increase the pH and inhibit the acidophilic microorganisms. Thus, optimisation of pH is recommended (Ilyas et al. 2010a). Optimal pH range is guided by the types of microorganism used for bioleaching (Table 1), and can be defined by conducting an optimisation study (Table 4). To avoid low dissolution yield, maintaining pH at optimum level by adding sulphuric acid is recommended (Chen et al. 2015; Roy et al. 2021a, 2021b). For the bioleaching employing fungi and cyanogenic bacteria pH of the culture medium can be adjusted my using HCl and NaOH (Naseri and Mousavi 2022). During bioleaching an automatic control unit via connected pH sensor can be utilised to maintain optimum pH at the constant level (Kremser et al. 2022).
Oxidation–reduction potential indicates the activities of the species and the flow of ions from metal-bearing-material to leaching media (Muddanna and Baral 2021; Roy et al. 2021a, 2021b). Muddanna and Baral, (2021) stated that oxidation–reduction potential ≥ 600 is good indicator for high Fe+3 for A. ferrooxidans. During acidophilic bioleaching oxidation–reduction potential above 400 mV indicates there is a high concentration of Fe+3 and high bacterial growth (Rawlings and Johnson 2007; Roy et al. 2021a, 2021b). Zare Tavakoli et al. (2017c) achieved 100% uranium dissolution from low grade uranium ore when oxidation–reduction potential was 550 mV by using A. ferrooxidans.
Solid concentration
Solid concentration is expresses as the term pulp density (% w/v) for shake flask and stirred tank. Low solid concentration (< 1%) provides efficient dissolution for both acidophilic and fungal bioleaching; however, this increases the cost of operation in large scale application (Srichandan et al. 2020). High solid concentration causes high buffering effect in acidophilic bioleaching, so acid addition into culture is needed to keep pH at favourable level during bioleaching (Chen and Lin 2010). In some optimisation studies, solid concentration was observed as the most important parameter (Gu et al. 2017; Nkulu et al. 2013). Like the other parameters, different pulp density can provide variations on leaching efficiency of different metals. For instance, Muddanna and Baral, (2021) achieved the highest La dissolution (83%) when 1% pulp density of spent fluid catalytic cracking catalyst was used, while the highest Ce dissolution (23%) was achieved when 5% and 7% pulp density was used. Amiri et al. (2011b) investigated the effect of one-step, two-step and spent medium step approaches using 1–5% (w/v) pulp density of spent hydrocracking catalyst by P. simplicissimum. Highest recovery of W, Al and Mo was achieved in two-step approach when 3% pulp density was used. Dissolution of W and Al were decreased from 67 to 37% and from 17 to 12% in the one-step approach when pulp density was increased from 1 to 5%.
When the spent medium step approach was used, the dissolution of all investigated metals was decreased as the pulp density increased. In different approach, Fe and Ni dissolution remained unchanged or decreased when pulp density increased. Overall results suggested that different metals were affected at different rate from selected bioleaching approach and pulp densities, but the two-step approach can provide high metal dissolution (> 90% W, Fe, Mo dissolution) at high pulp density (> 1%). Pulp density can be optimised up to 20% (w/v) for agitated tank reactors (Natarajan 2018) (Fig. 2). Higher solid liquid ratio (> 20% w/v) can be used for column, heap, and dump bioleaching. For instance, Mousavi et al. (2006) achieved 72% zinc dissolution by using 22 L of culture for 410 kg of low-grade sphalerite ore in column bioleaching after 120 days. BIOPRO™ used 117,200 m3 capacity solution pond to bioleach 810,000 tons of refractory sulfidic ore in commercial heap process (Rawlings and Johnson 2007).
Energy source choice and concentration
The type of energy source is dependent on the selected microorganisms (Table 5). The choice of energy source is also important for the material that should be treated. Some oxidic materials such as waste incineration ashes and slags require the action of both Fe3+ and H2SO2 (Kremser et al. 2021). Wang et al. (2015) discussed that during bioleaching of Pb/Zn smelting slag Indium (In) dissolution was both acid dissolution by biogenic H2SO4 and oxidation/reduction by Fe2+/Fe3+, whereas Zn, Cd and Pb followed acid dissolution mechanism. Others for instance spent Li-Ion batteries or waste printed circuit boards require Fe2+, Fe3+ or H2SO4 only (Chen et al. 2015; Ghassa et al. 2020; Roy et al. 2021a, 2021b).
Optimisation of energy source concentration ranging between 0.1 and 20% (w/v) can be recommended to achieve a high bioleaching yield and to make the process economical (Gu et al. 2017; Mousavi et al. 2006; Xu and Ting 2004). Pathak et al. (2009) investigated the effects of ferric ion concentration on bioleaching of sewage sludge using two different energy sources, ammonium ferrous sulphate and ferrous sulphate, for iron-oxidising microorganisms. Using ferrous sulphate provided better metal bioleaching yield, 64% Cu, 58% Ni, 76% Zn, 52% Cr, than ammonium ferrous sulphate, 56% Cu, 48% Ni, 68% Zn, 42% Cr.
Microbial acclimatisation and inoculum concentration
Before starting the bioleaching process, it is recommended to acclimatise the selected microorganism(s) with the metal-bearing-material to enhance their metal toxicity tolerance (Amiri et al. 2011b). This is typically performed by culturing the microbes in liquid medium until the log phase is reached. Then small amounts (0.2% w/v) of solid material are added into the culture (Chen et al. 2015). Once the cells reach again the log phase, sub-culturing is performed fir increased material concentration. This step can be repeated as necessary to reach the desired solid:liquid ratio (Muddanna and Baral 2021). Acclimatisation can also be performed on petri dish using a growth medium containing metal ions for fungi (Amiri et al. 2011b). Several bioleaching studies confirmed that acclimatised microorganisms provided higher metal dissolution than non-acclimatised microorganisms. For example, Abhilash et al. (2013) acclimatised A. ferrooxidans and L. ferrooxidans up to 5% (w/v) sample concentration. Once both species were acclimatised, they were able to solubilise 57% and 66%, respectively of uranium from low-grade apatite rich uraninite ore. Muddanna and Baral, (2021) adapted A. ferrooxidans up to 20% (w/v) spent fluid catalytic cracking catalyst. They stated that acclimatized microorganisms ensured higher bioleaching yield than non-acclimatised culture. Chen et al. (2015) adapted A. ferrooxidans to waste printed circuit boards from 0.2% (w/v) to 3.5% (w/v) and achieved 94.8% Cu recovery after 28 days. Ilyas et al. (2010b) operated for 2 years a column bioleaching process by using adapted mixed culture of Sulfobacilllus thermosulfidooxidans and Thermoplasma acidophilum. They were able to recover 80% Zn, 64% Al, 86% Cu and 74% Ni from 10 kilos of electronic scrap.
Inoculum concentration is also an important parameter affecting leaching time and dissolution yield (Zare Tavakoli et al. 2017b). Several researchers tried to optimise inoculum concentration from 0.1% up to 30% (v/v) for different metal bearing material (Ilyas et al. 2012; Mo et al. 2019; Zare Tavakoli et al. 2017b; Table S8; Table S10). When the effect of inoculum concentration was evaluated with the one-factor-at-a-time methodology, it appeared that an increase in the inoculum concentration had positive effect on the bioleaching performance (Hosseinzadeh et al. 2021; Jagannath et al. 2017). When multivariable optimisation method was used (e.g., Plackett − Burman factorial design, central composite design), inoculum concentration was usually found to be the second, third or fourth important factor. So that, it mostly come after pH, solid concentration, and energy source concentration by the order of importance (Arshadi et al. 2019; Zare Tavakoli et al. 2017b; Xu and Ting 2004). Overall, several studies revealed that increase in inoculation percent had positive effect on bioleaching yield; however, higher concentration than optimum value caused the precipitation of some metal ions in reactor during bioleaching of low-grade ore or e-waste (Jagannath et al. 2017; Zare Tavakoli et al. 2017b). Therefore, inoculum concentration is suggested as an optimisation parameter for future works due to it may have a positive effect on bioleaching yield and the cost of operation. Since to reach a high inoculation percent high energy source needs to be used, it will also increase the operational cost.
Aeration rate and temperature
Aeration provides an efficient transfer of O2 and CO2 for microorganism in both agitated and percolated systems (Srichandan et al. 2020). 1.5–4 mg/L dissolved oxygen concentration is recommended to be managed inside the reactor (Rawlings and Johnson 2007). Ilyas and Lee, (2014) optimised aeration rate between 0.07–1 L/min for a stirred bioleaching tank inoculated with Sulfobacillus thermosulfidooxidans. They found that increasing aeration rate from 0.07 to 0.5 L/min accelerated the leaching rate for Al, Cu, Zn, Ni and reduced the lag time of the bacterial culture approximately 50%. However, increasing the aeration rate more than 0.5 L/min adversely affected the bacterial growth and resulted in decreased metal dissolution. Zare Tavakoli et al. (2017a) tested different air flows ranging between 50 and 250 L/h for 7.5 cm diameter column for U bioleaching. They reported that the optimum value was 100 L/h (1667 ml/min) to achieve 100% of U bioleaching. Higher air flow than 100 L/h caused decrease in bioleaching yield due to bacterial growth adversely affected by increasing excessive turbulence, shear stress and cellular attrition in column. Chen et al. (2015) used a 20 L/min of aeration rate for a column which has an internal diameter of 6 cm. They recovered 95% Cu form waste printed circuit boards. Natarajan (2018) reported the typical aeration rates for a heap operation are 0.1–0.5 m3/m2h.
Maintaining temperature at desired level based on employed microorganisms is important to achieve efficient metal dissolution (Table 1) (Mousavi et al. 2006; Srichandan et al. 2020). Irrigation and aeration rate influence the temperature in percolation systems (Mousavi et al. 2006). Due to bioleaching is an exothermic process and ores have diverse indigenous organisms, the dominant microbial community can change with time in heap bioleaching (Natarajan 2018). Stirred tanks are designed to maintain the reactor temperatures at desired levels to cope with exothermic heat generation, thus they provide more controlled environment than heap (Natarajan 2018). To avoid significant temperature changes in column bioleaching, using water bath for reservoir and water jacket around the column can be preferred (Ilyas et al. 2010b).
Agitation speed and irrigation rate
While agitation rate is a specific parameter for shake flask and stirred bioreactor, irrigation rate is a specific parameter of the percolated systems e.g., column, heap, dump (Natarajan 2018). Good mixing condition is needed to ensure gas–liquid-solid transfer and heat distribution (Natarajan 2018). Optimum agitation speed will be depended on the type and design of the reactor (Amiri et al. 2011b; Eisapour et al. 2013) and the target metal in the metal-bearing-material (Nkulu et al. 2013). High metal dissolution (> 90%) can be achieved when agitation speed is set up between 120–240 rpm for shake flask (Amiri et al. 2011b; Işıldar et al. 2016), and 150–600 rpm for stirred tank bioleaching (Eisapour et al. 2013; Kremser et al. 2020; Pathak et al. 2015). For example, Nkulu et al. (2013) optimised agitation speed between 200 and 400 rpm along with temperature, pH, leaching duration and pulp density for bioleaching of a polymetallic flotation concentrate by using acidophiles. As a result, agitation speed appeared as the second most important parameter for the Co leaching and optimum value was 250 rpm. On the other hand, for Ni and Cu, it was the least important factor, and the optimum values were 300 and 350 rpm, respectively. Ilyas et al. (2010a) investigated the best operational conditions for bioleaching of metal ions from low grade sulphide ore by optimising agitation speed along with four other parameters. The agitation speed was the third significant parameter for S. thermosulfidooxidans, and the optimum value was 180 rpm to achieve 72 Zn%, 68% Co, 78% Cu, 81% Ni and 70% Fe dissolution.
In terms of irrigation rate, Mousavi et al. (2006) reported that liquid irrigation rate is an important operational parameter to increase the bioleaching kinetic, and it must be optimised. Like agitation speed there is no clear trend for irrigation rate as it will be depended on the type and design of the reactor and the metal-bearing-material (Chen et al. 2015; Zare Tavakoli et al. 2017a, b). Typical irrigation rates are 5–20 L/m2h for heap and 5–40 L/m2h for column (Natarajan 2018). Mousavi et al. (2006) tested three irrigation rates (5, 10, 20 L/m2h), for A. ferrooxidans and Sulfobacillus. The lowest irrigation rate provided higher Zn dissolution, > 70%, from the low-grade sphalerite ore while the highest irrigation rate provided only < 50% Zn dissolution for both microorganisms. In another study, Chen et al.,(2015) used an irrigation rate of 40 ml/min by recirculating the leaching solution. They suggested that increasing the cycling velocity (> 40 ml/min) of the leached solution can accelerate the kinetics of bioleaching yield in the 6 cm diameter column reactor. In contrast, Zare Tavakoli et al. (2017a, b, c) evaluated two irrigation rates (0.25 and 2.25 ml/min) along with seven independent parameters, aeration rate, the concentration of initial ferrous, pH, temperature, inoculation percent and particle size, in column (no circulation) bioleaching for uranium recovery. They found that that irrigation rate was not a statistically important parameter.
Catalyst
Bioleaching performance can be enhanced by the addition of catalysts into the bioleaching medium, such as silver ions (Ag+), graphene, biochar. For example, Zeng et al. (2013) investigated the effect of silver ions (Ag+) on the solubilisation of cobalt (Co) from spent lithium batteries by using A. ferrooxidans. 98% Co recovery was achieved after 7 days of bioleaching, whereas Co solubilisation was only 43% in the absence of Ag+. Wang et al. (2016) showed that the addition of biochar to e-waste contribute to the complete solubilisation of copper (Cu) in 2 days, while only 80% was achieved without it. Gu et al. (2017) explored the catalytic effect of graphene on the dissolution of copper from waste printed circuit board by using A. ferrooxidans. Copper dissolution was enhanced by 10% compared to the control. Based on the conducted studies it can be assumed that utilisation of catalyst can provide higher bioleaching yields. However, understanding the additional materials effect on bioleaching kinetic can be difficult. Thus, it can be suggested that once the main operational parameters, which are pH, solid concentration, energy source concentration, irrigation rate/agitation speed, are optimized, the catalyst addition can be evaluated to increase further the bioleaching yield.
Industrial application needs and perspectives
Bioleaching is already performed on industrial scale for various types of ores to extract copper and gold by using acidophiles (Demergasso et al. 2010; Galleguillos et al. 2008; Gericke et al. 2009; Halinen et al. 2009; Soto et al. 2013). Heap and dump as well as agitated tank are the most preferred operation types for industrial scale applications. However, in large scale changes in pH and microbial community structure due to poor management of operational parameters e.g., air flow and CO2 availability lead to a decreased leaching rate (Gao et al. 2021; Marín et al. 2021). Recent advances in biomining such as gene libraries, Polymerase Chain Reaction (PCR)-based techniques are promising for a better understanding of the microbial dynamics (Marín et al. 2021; Natarajan 2018). Besides, optimisation of new influencing parameters such as micro-cracks (Chen et al. 2020) and characterisation of novel microorganisms (Natarajan 2018) may help to overcome industrial scale limitations. In-situ bioleaching is successfully applied for Cu currently. Yin et al. (2018) reported some underground in-situ bioleaching operations for copper in China, achieving over 95% recovery in Tongguanshan Copper Mine in 1980 and > 500 t/a in Zhongtiaoshan Copper Mine in 2000. In terms of uranium, a few in-situ bioleaching studies have been reported with 50–70% efficiency (Abhilash et al. 2015). Lots of research are promising to be in-situ bioleaching widely applicable in future (Götze et al. 2022; Huang et al. 2018; Laurent et al. 2019) and to reduce its possible environmental impacts (Ballerstedt et al. 2017).
To the best of the authors knowledge there is no commercial bioleaching applications for other metal-bearing-wastes than ores (Gao et al. 2021; Kaksonen et al. 2020; Natarajan 2018). Several promising intermediate scale, which are column and stirred tank, bioleaching studies have been published for various types of metal-bearing-material (Tables 2, 3, and 4), yet they are not commercialised (Gao et al. 2021; Srichandan et al. 2019). Although lab bioleaching experiments revealed high bioleaching yields for some metals (> 80% Al, Cu, V, Zn, Ni) at low pulp densities (≤ 5% w/v), research needs to be intensified to understand and accelerate the bioleaching kinetics towards industrial scale (Chen et al. 2015; Nkulu et al. 2013; Pathak et al. 2019). One limitation about acidophiles that during bioleaching leached metal ions in the solution can precipitate due to jarosite formation (Baniasadi et al. 2019). This adversely affects the recovery and inhibits the bioleaching. To maintain jarosite formation optimisation of temperature, pH and ferrous iron concentration as well as continuous pH adjustment is recommended (Opara et al. 2022). In many bioleaching studies one-factor-at-a-time methodology was used to identify the optimum value for these parameters. However, rather than using one-factor-at-a-time methodology, multiparameter optimisation is recommended to understand the interactions between parameters and to identify optimal conditions in the process (Niu et al. 2016). According to this, optimisation can be performed as two-phases process. First, analysing of process parameters using a screening method, such as Plackett–Burman, this will allow researcher to understand the most influencing factors as a first insight (Amiri et al. 2011a; Zare Tavakoli et al. 2017b). Then, further optimisation can be performed with response surface methods, such as a central composite design or Box–Behnken, or Taguchi orthogonal array design to find optimum values for the identified most influencing parameters (Amiri et al. 2011a; Jalali et al. 2019; Mo et al. 2019; Nkulu et al. 2013). Here, we propose a two-phase optimisation route to achieve a high metal recovery yield from small to large scale bioleaching operation (Fig. 4) (see Supplementary Material Document 2 for the detailed flow chart). Our procedure modified from Potysz et al. (2018) the “flowsheet to develop optimized bioleaching protocol for slags” and improved and generalized for all types of metal-bearing-materials. Besides, analysing metals leaching kinetics provide broader understanding of the leaching behaviours of different metals during bioleaching (Amiri et al. 2012; Chen et al. 2015; Pathak et al. 2019). Therefore, selective metal solubilisation can be possible (Nkulu et al. 2013). In addition, there are limited studies about selective recovery of target metals from the biolixiviant after bioleaching (Kremser et al. 2022). In conclusion, more collaborative research with chemist, biologist and metallurgist is needed to make bioleaching of metal-bearing-wastes commercially feasible (Holmes 2008; Roy et al. 2021a, 2021b).
Two-phase optimisation routes for bioleaching of metal-bearing materials. Flow chart covers characterisation and pre-treatment of metal-bearing materials, microorganism selection and acclimatisation followed by first phase of optimisation which is on shake flask scale. In the second phase of optimisation, intermediate scale bioleaching is suggested using column or bioreactor. MBM stands for metal-bearing material. Modified and improved from Potysz et al. (2018). See Supplementary Material Document 2 for the detailed flow chart
To recover metals from some metal-bearing-materials, such as e-waste, biohydrometallurgy requires low installation and operation costs and provides more environmentally friendly process when compared to pyrometallurgy and hydrometallurgy (Arya and Kumar 2020b; Baniasadi et al. 2019). Applying life cycle assessment and techno economic analysis are recommended by several researchers as it would be very helpful to quantifying the environmental impact of the process (Baniasadi et al. 2021; Sadhukhan, Ng and Hernandez 2014; Villares et al. 2016). Some small-scale life cycle assessment provided valuable insights about the impacts of the bioleaching process as well as guide to researchers to identify potential hotspots to reduce the process overall impact. For instance, Sun et al. (2016) found that during bioleaching of Zn-Mn batteries, cutting and crushing the batteries have the highest environmental impact on the human toxicity and marine ecotoxicity. In another techno-economic analysis life cycle assessment showed that during bioleaching of fluidized catalytic cracking catalysts 44% of the cost was due to the using of raw energy source for microorganism, and raw energy source and electricity requirement was responsible for the largest proportion of the environmental impact (Thompson et al. 2018). Researchers suggested that utilisation of alternative energy sources, for example agricultural waste or real organic wastewater for heterotrophs, and optimisation of energy source can reduce the environmental impact of the process (Baniasadi et al. 2021; Gavrilescu 2022; Jin et al. 2019). Moreover, an ex-ante life cycle assessment study, based on small scale laboratory and pilot bioleaching study results, can help to predict the environmental impact of a commercial scale bioleaching application on the early development stage (Villares et al. 2016).
Conclusion
Bioleaching has emerged as a promising cost-effective and environmentally friendly alternative for recovering metals from complex metal-bearing materials. Acidophilic bacteria and fungi are key microorganisms that play an important role in metal dissolution through specific mechanisms, and recent advances in gene libraries and PCR-based techniques offer effective on-site monitoring and better understanding of microbe-mineral/waste interactions. Although bioleaching has been successfully applied at a commercial scale for various types of low-grade ores and tailings, slow dissolution kinetics remain a challenge. To address this, multivariable optimization methods such as orthogonal array design, Plackett–Burman, and response surface analysis should be used to achieve higher bioleaching yields. Solid concentration, pH, energy source concentration, and particle size are the most influential parameters, and analysing reaction kinetics is essential to enable selective metal extraction. Furthermore, we recommend that more pilot studies and collaborative research involving chemists, biologists, and metallurgists working together should be conducted to scale up bioleaching for industrial use, especially for other metal-bearing materials such as e-waste, metallurgical sludge, and dust, spent catalysts, and fly ash. Finally, applying life cycle assessment and techno-economic assessment can help evaluate and reduce the environmental impact and cost of the bioleaching process. Our proposed optimisation flow chart can help achieve high bioleaching yield from general metal-bearing materials from laboratory to pilot/large scale operations. Implementing these recommendations can lead to the continued advancement of the field, providing a sustainable solution for metal recovery that minimises environmental impact.
Data availability
All the data supporting this study are included within the article and/or supporting materials.
Change history
02 August 2023
The missed supplementary information has been included.
References
Abhilash, Pandey BD (2013) Microbially assisted leaching of uranium - A review. Miner Process Extr Metall Rev 34:81–113. https://doi.org/10.1080/08827508.2011.635731
Abhilash, Mehta KD, Kumar V, Pandey BD, Tamrakar PK (2010) Column bioleaching of a low-grade silicate ore of uranium. Miner Process Extr Metall Rev 31:224–235. https://doi.org/10.1080/08827508.2010.483380
Abhilash, Pandey BD, Natarajan KA (Eds) (2015) Microbiology for minerals, metals, materials and the environment, 1st ed. CRC Press .pp 608. https://doi.org/10.1201/b18124〹
Akcil A, Erust C, Gahan CS, Ozgun M, Sahin M, Tuncuk A (2015) Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants – A review. Waste Manage 45:258–271. https://doi.org/10.1016/j.wasman.2015.01.017
Amiri F, Mousavi SM, Yaghmaei S (2011a) Enhancement of bioleaching of a spent Ni/Mo hydroprocessing catalyst by Penicillium simplicissimum. Sep Purif Technol 80:566–576. https://doi.org/10.1016/j.seppur.2011.06.012
Amiri F, Yaghmaei S, Mousavi SM (2011b) Bioleaching of tungsten-rich spent hydrocracking catalyst using Penicillium simplicissimum. Biores Technol 102:1567–1573. https://doi.org/10.1016/j.biortech.2010.08.087
Amiri F, Mousavi SM, Yaghmaei S, Barati M (2012) Bioleaching kinetics of a spent refinery catalyst using Aspergillus Niger at optimal conditions. Biochem Eng J 67:208–217. https://doi.org/10.1016/j.bej.2012.06.011
Arab B, Hassanpour F, Arshadi M, Yaghmaei S, Hamedi J (2020) Optimized bioleaching of copper by indigenous cyanogenic bacteria isolated from the landfill of e-waste. J Environ Manag 261:110124. https://doi.org/10.1016/j.jenvman.2020.110124
Arshadi M, Nili S, Yaghmaei S (2019) Ni and Cu recovery by bioleaching from the printed circuit boards of mobile phones in non-conventional medium. J Environ Manag 250:109502. https://doi.org/10.1016/j.jenvman.2019.109502
Arya S, Kumar S (2020a) Bioleaching: urban mining option to curb the menace of E-waste challenge. Bioengineered 11:640–660. https://doi.org/10.1080/21655979.2020.1775988
Arya S, Kumar S (2020b) E-waste in India at a glance: Current trends, regulations, challenges and management strategies. J Clean Prod 271:122707. https://doi.org/10.1016/j.jclepro.2020.122707
Asghari I, Mousavi SM, Amiri F, Tavassoli S (2013) Bioleaching of spent refinery catalysts: a review. J Ind Eng Chem 19:1069–1081. https://doi.org/10.1016/j.jiec.2012.12.005
Ballerstedt H, Pakostova E, Johnson DB, Schippers A (2017) Approaches for eliminating bacteria introduced during in situ bioleaching of fractured sulfidic ores in deep subsurface. Solid State Phenom 262:70–74. https://doi.org/10.4028/www.scientific.net/SSP.262.70
Baniasadi M, Vakilchap F, Bahaloo-Horeh N, Mousavi SM, Farnaud S (2019) Advances in bioleaching as a sustainable method for metal recovery from e-waste: a review. J Ind Eng Chem 76:75–90. https://doi.org/10.1016/j.jiec.2019.03.047
Baniasadi M, Graves JE, Ray DA, de Silva AL, Renshaw D, Farnaud S (2021) Closed-loop recycling of copper from waste printed circuit boards using bioleaching and electrowinning processes. Waste Biomass Valorization 12:3125–3136. https://doi.org/10.1007/s12649-020-01128-9
Bayat O, Sever E, Bayat B, Arslan V, Poole C (2008) Bioleaching of zinc and iron from steel plant waste using Acidithiobacillus ferrooxidans. Appl Biochem Biotechnol 152:117–126. https://doi.org/10.1007/s12010-008-8257-5
Benzal E, Solé M, Lao C, Gamisans X, Dorado AD (2020) Elemental copper recovery from e-wastes mediated with a two-step bioleaching process. Waste Biomass Valorization 11:5457–5465. https://doi.org/10.1007/s12649-020-01040-2
Bhatti TM (2015) Bioleaching of organic carbon rich polymetallic black shale. Hydrometallurgy 157:246–255. https://doi.org/10.1016/j.hydromet.2015.08.012
Binnemans K, Jones PT, Manjón Fernández Á, Masaguer Torres V (2020) Hydrometallurgical processes for the recovery of metals from steel industry by-products: a critical review. J Sustain Metall 6:505–540. https://doi.org/10.1007/s40831-020-00306-2
BIT (2019), BIT II - Bioeconomy in Italy: a new bioeconomy strategy for a sustainable Italy, Italy available at https://knowledge4policy.ec.europa.eu/publication/bit-ii-bioeconomy-italy-new-bioeconomy-strategy-sustainable-italy_en (accessed on 23/04/2023)
Brandl H, Lehmann S, Faramarzi MA, Martinelli D (2008) Biomobilization of silver, gold, and platinum from solid waste materials by HCN-forming microorganisms. Hydrometallurgy 94:14–17. https://doi.org/10.1016/j.hydromet.2008.05.016
Chen SY, Lin PL (2010) Optimization of operating parameters for the metal bioleaching process of contaminated soil. Sep Purif Technol 71:178–185. https://doi.org/10.1016/j.seppur.2009.11.018
Chen S, Yang Y, Liu C, Dong F, Liu B (2015) Column bioleaching copper and its kinetics of waste printed circuit boards (WPCBs) by Acidithiobacillus ferrooxidans. Chemosphere 141:162–168. https://doi.org/10.1016/j.chemosphere.2015.06.082
Chen J, Tang D, Zhong S, Zhong W, Li B (2020) The influence of micro-cracks on copper extraction by bioleaching. Hydrometallurgy 191:105243. https://doi.org/10.1016/j.hydromet.2019.105243
Chu H, Qian C, Tian B, Qi S, Wang J, Xin B (2022) Pyrometallurgy coupling bioleaching for recycling of waste printed circuit boards. Resour Conserv Recycl 178:106018. https://doi.org/10.1016/j.resconrec.2021.106018
Costa OYA, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol 9:1636. https://doi.org/10.3389/fmicb.2018.01636
Demergasso C, Galleguillos F, Soto P, Serón M, Iturriaga V (2010) Microbial succession during a heap bioleaching cycle of low grade copper sulfides: does this knowledge mean a real input for industrial process design and control? Hydrometallurgy 104:382–390. https://doi.org/10.1016/j.hydromet.2010.04.016
Dusengemungu L, Kasali G, Gwanama C, Mubemba B (2021) Overview of fungal bioleaching of metals. Environ Adv 5:100083. https://doi.org/10.1016/j.envadv.2021.100083
Dutta D, Rautela R, Gujjala LKS, Kundu D, Sharma P, Tembhare M, Kumar S (2023) A review on recovery processes of metals from E-waste: a green perspective. Sci Total Environ 859:160391. https://doi.org/10.1016/j.scitotenv.2022.160391
Eisapour M, Keshtkar A, Moosavian MA, Rashidi A (2013) Bioleaching of uranium in batch stirred tank reactor: process optimization using Box-Behnken design. Ann Nucl Energy 54:245–250. https://doi.org/10.1016/j.anucene.2012.11.006
Faramarzi MA, Mogharabi-Manzari M, Brandl H (2020) Bioleaching of metals from wastes and low-grade sources by HCN-forming microorganisms. Hydrometallurgy 191:105228. https://doi.org/10.1016/j.hydromet.2019.105228
Figueroa-Estrada JC, Aguilar-López R, Rodríguez-Vázquez R, Neria-González MI (2020) Bioleaching for the extraction of metals from sulfide ores using a new chemolithoautotrophic bacterium. Hydrometallurgy 197:105445. https://doi.org/10.1016/j.hydromet.2020.105445
Galleguillos P, Remonsellez F, Galleguillos F, Guiliani N, Castillo D, Demergasso C (2008) Identification of differentially expressed genes in an industrial bioleaching heap processing low-grade copper sulphide ore elucidated by RNA arbitrarily primed polymerase chain reaction. Hydrometallurgy 94:148–154. https://doi.org/10.1016/j.hydromet.2008.05.031
Gao X, Jiang L, Mao Y, Yao B, Jiang P (2021) Progress, challenges, and perspectives of bioleaching for recovering heavy metals from mine tailings. Adsorpt Sci Technol 2021:1–13. https://doi.org/10.1155/2021/9941979
Gavrilescu M (2022) Microbial recovery of critical metals from secondary sources. Bioresour Technol 344:126208. https://doi.org/10.1016/j.biortech.2021.126208
Gericke M, Neale JW, Van Staden PJ (2009) A Mintek perspective of the past 25 years in minerals bioleaching. J Southern African Institute Mining Metall 109:567–585
Ghassa S, Farzanegan A, Gharabaghi M, Abdollahi H (2020) Novel bioleaching of waste lithium ion batteries by mixed moderate thermophilic microorganisms, using iron scrap as energy source and reducing agent. Hydrometallurgy 197:105465. https://doi.org/10.1016/j.hydromet.2020.105465
Gholami RM, Borghei SM, Mousavi SM (2011) Bacterial leaching of a spent Mo-Co-Ni refinery catalyst using Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 106:26–31. https://doi.org/10.1016/j.hydromet.2010.11.011
Gomes HI, Funari V, Mayes WM, Rogerson M, Prior TJ (2018) Recovery of Al, Cr and V from steel slag by bioleaching: batch and column experiments. J Environ Manage 222:30–36. https://doi.org/10.1016/j.jenvman.2018.05.056
Götze K, Haseneder R, Braeuer AS (2022) Investigations on strategic element recovery by an underground membrane pilot plant from in-situ extracted bioleaching solutions. Minerals 12:46. https://doi.org/10.3390/min12010046
Gu W, Bai J, Dong B, Zhuang X, Zhao J, Zhang C, Wang J, Shih K (2017) Catalytic effect of graphene in bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 171:172–178. https://doi.org/10.1016/j.hydromet.2017.05.012
Gutiérrez-Gutiérrez SC, Coulon F, Jiang Y, Wagland S (2015) Rare earth elements and critical metal content of extracted landfilled material and potential recovery opportunities. Waste Manage 42:128–136. https://doi.org/10.1016/j.wasman.2015.04.024
Halinen AK, Rahunen N, Kaksonen AH, Puhakka JA (2009) Heap bioleaching of a complex sulfide ore: part II. Effect of temperature on base metal extraction and bacterial compositions. Hydrometallurgy 98:101–107. https://doi.org/10.1016/j.hydromet.2009.04.004
He ZG, Yang YP, Zhou S, Hu YH, Zhong H (2014) Effect of pyrite, elemental sulfur and ferrous ions on EPS production by metal sulfide bioleaching microbes. Trans Nonferrous Met Soc China 24:1171–1178. https://doi.org/10.1016/S1003-6326(14)63176-9
Holmes DS (2008) Review of international biohydrometallurgy symposium, Frankfurt, 2007. Hydrometallurgy 92:69–72. https://doi.org/10.1016/j.hydromet.2008.01.003
Hosseinzadeh F, Rastegar SO, Ashengroph M (2021) Bioleaching of rare earth elements from spent automobile catalyst as pretreatment method to improve Pt and Pd recovery: process optimization and kinetic study. Process Biochem 105:1–7. https://doi.org/10.1016/j.procbio.2021.03.022
Huang C, Qin C, Feng X, Liu X, Yin H, Jiang L, Liang Y, Liu H, Tao J (2018) Chalcopyrite bioleaching of an in situ leaching system by introducing different functional oxidizers. RSC Adv 8:37040. https://doi.org/10.1039/C8RA07085G
IEA (2021) The role of critical minerals in clean energy transitions. OECD Publishing, Paris. https://doi.org/10.1787/f262b91c-en
Ilyas S, Lee JC (2014) Bioleaching of metals from electronic scrap in a stirred tank reactor. Hydrometallurgy 149:50–62. https://doi.org/10.1016/j.hydromet.2014.07.004
Ilyas S, Bhatti HN, Bhatti IA, Sheikh MA, Ghauri MA (2010a) Bioleaching of metal ions from low grade sulphide ore: process optimization by using orthogonal experimental array design. African J Biotechnol 9:2801–2810
Ilyas S, Ruan C, Bhatti HN, Ghauri MA, Anwar MA (2010b) Column bioleaching of metals from electronic scrap. Hydrometallurgy 101:135–140. https://doi.org/10.1016/j.hydromet.2009.12.007
Ilyas S, Chi R, Lee JC, Bhatti HN (2012) One step bioleaching of sulphide ore with low concentration of arsenic by Aspergillus niger and Taguchi orthogonal array optimization. Chin J Chem Eng 20:923–929. https://doi.org/10.1016/S1004-9541(12)60419-4
Işıldar A, van de Vossenberg J, Rene ER, van Hullebusch ED, Lens PNL (2016) Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manage 57:149–157. https://doi.org/10.1016/j.wasman.2015.11.033
Işıldar A, van Hullebusch ED, Lenz M, Du Laing G, Marra A, Cesaro A, Panda S, Akcil A, Kucuker MA, Kuchta K (2019) Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE) – A review. J Hazard Mater 362:467–481. https://doi.org/10.1016/j.jhazmat.2018.08.050
Jagannath A, Vidya Shetty K, Saidutta MB (2017) Bioleaching of copper from electronic waste using Acinetobacter sp. Cr B2 in a pulsed plate column operated in batch and sequential batch mode. J Environ Chem Eng 5:1599–1607. https://doi.org/10.1016/j.jece.2017.02.023
Jalali F, Fakhari J, Zolfaghari A (2019) Response surface modeling for lab-scale column bioleaching of low-grade uranium ore using a new isolated strain of Acidithiobacillus Ferridurans. Hydrometallurgy 185:194–203. https://doi.org/10.1016/j.hydromet.2019.02.014
Jin H, Reed DW, Thompson VS, Fujita Y, Jiao Y, Crain-Zamora M, Fisher J, Scalzone K, Griffel M, Hartley D, Sutherland JW (2019) Sustainable bioleaching of rare earth elements from industrial waste materials using agricultural wastes. ACS Sustain Chem Eng 7:15311–15319. https://doi.org/10.1021/acssuschemeng.9b02584
Jones PT, Geysen D, Tielemans Y, Van Passel S, Pontikes Y, Blanpain B, Quaghebeur M, Hoekstra N (2013) Enhanced Landfill Mining in view of multiple resource recovery: A critical review. J Clean Prod 55:45–55. https://doi.org/10.1016/j.jclepro.2012.05.021
Kaksonen AH, Lakaniemi AM, Tuovinen OH (2020) Acid and ferric sulfate bioleaching of uranium ores: a review. J Clean Prod 264:121586. https://doi.org/10.1016/j.jclepro.2020.121586
Keshavarz S, Faraji F, Rashchi F, Mokmeli M (2021) Bioleaching of manganese from a low-grade pyrolusite ore using Aspergillus niger: Process optimization and kinetic studies’. J Environ Manag 285:112153. https://doi.org/10.1016/j.jenvman.2021.112153
Kremser K, Thallner S, Schoen H, Weiss S, Hemmelmair C, Schnitzhofer W, Aldrian A, Guebitz GM (2020) Stirred-tank and heap-bioleaching of shredder-light-fractions (SLF) by acidophilic bacteria. Hydrometallurgy 193:105315. https://doi.org/10.1016/j.hydromet.2020.105315
Kremser K, Thallner S, Strbik D, Spiess S, Kucera J, Vaculovic T, Vsiansky D, Haberbauer M, Mandl M, Guebitz GM (2021) Leachability of metals from waste incineration residues by iron- and sulfur-oxidizing bacteria. J Environ Manag 280:111734. https://doi.org/10.1016/j.jenvman.2020.111734
Kremser K, Thallner S, Spiess S, Kucera J, Vaculovic T, Všianský D, Haberbauer M, Guebitz GM (2022) Bioleaching and selective precipitation for metal recovery from basic oxygen furnace slag. Processes 10:576. https://doi.org/10.3390/pr10030576
Kumar A, Saini HS, Kumar S (2018) Bioleaching of gold and silver from waste printed circuit boards by Pseudomonas balearica SAE1 isolated from an e-waste recycling facility. Curr Microbiol 75:194–201. https://doi.org/10.1007/s00284-017-1365-0
Laurent G, Izart C, Lechenard B, Golfier F, Marion P, Collon P, Truche L, Royer JJ, Filippov L (2019) Numerical modelling of column experiments to investigate in-situ bioleaching as an alternative mining technology. Hydrometallurgy 188:272–290. https://doi.org/10.1016/j.hydromet.2019.07.002
Lee H, Coulon F, Beriro DJ, Wagland ST (2022a) Recovering metal(loids) and rare earth elements from closed landfill sites without excavation: leachate recirculation opportunities and challenges. Chemosphere 292:133418. https://doi.org/10.1016/j.chemosphere.2021.133418
Lee H, Coulon F, Wagland ST (2022b) Influence of pH, depth and humic acid on metal and metalloids recovery from municipal solid waste landfills. Sci Total Environ 806:150332. https://doi.org/10.1016/j.scitotenv.2021.150332
Liu R, Li J, Ge Z (2016) Review on Chromobacterium Violaceum for gold bioleaching from e-waste. Procedia Environ Sci 31:947–953. https://doi.org/10.1016/j.proenv.2016.02.119
Ma N (2016) Recycling of basic oxygen furnace steelmaking dust by in-process separation of zinc from the dust. J Clean Prod 112:4497–4504. https://doi.org/10.1016/j.jclepro.2015.07.009
Ma L, Huang S, Wu P, Xiong J, Wang H, Liao H, Liu X (2021) The interaction of acidophiles driving community functional responses to the re-inoculated chalcopyrite bioleaching process. Sci Total Environ 798:149186. https://doi.org/10.1016/j.scitotenv.2021.149186
Maluckov BS (2017) The catalytic role of acidithiobacillus ferrooxidans for metals extraction from mining-metallurgical resource. Biodiv Int J 1:109–119. https://doi.org/10.15406/bij.2017.01.00017
Marín S, Cortés M, Acosta M, Delgado K, Escuti C, Ayma D, Demergasso C (2021) From laboratory towards industrial operation: biomarkers for acidophilic metabolic activity in bioleaching systems. Genes 12:474. https://doi.org/10.3390/genes12040474
Merli G, Becci A, Amato A (2022) Recovery of precious metals from printed circuit boards by cyanogenic bacteria: optimization of cyanide production by statistical analysis. J Environ Chem Eng 10:107495. https://doi.org/10.1016/j.jece.2022.107495
Mikoda B, Potysz A, Kmiecik E (2019) Bacterial leaching of critical metal values from Polish copper metallurgical slags using Acidithiobacillus thiooxidans. J Environ Manage 236:436–445. https://doi.org/10.1016/j.jenvman.2019.02.032
Mishra D, Kim D-J, Ahn J-G, Rhee Y-H (2005) Bioleaching: a microbial process of metal recovery; a review. Met Mater Int 11:249–256. https://doi.org/10.1007/bf03027450
Mishra G, Jha R, Meshram A, Singh KK (2022) A review on recycling of lithium-ion batteries to recover critical metals. J Environ Chem Eng 10:108534. https://doi.org/10.1016/j.jece.2022.108534
Mo X, Li X, Wen J (2019) Optimization of bioleaching of fluoride-bearing uranium ores by response surface methodology. J Radioanal Nucl Chem 321:579–590. https://doi.org/10.1007/s10967-019-06594-7
Moazzam P, Boroumand Y, Rabiei P, Baghbaderani SS, Mokarian P, Mohagheghian F, Mohammed LJ, Razmjou A (2021) Lithium bioleaching: an emerging approach for the recovery of Li from spent lithium ion batteries. Chemosphere 277:130196. https://doi.org/10.1016/j.chemosphere.2021.130196
Mousavi SM, Jafari A, Yaghmaei S, Vossoughi M, Roostaazad R (2006) Bioleaching of low-grade sphalerite using a column reactor. Hydrometallurgy 82:75–82. https://doi.org/10.1016/j.hydromet.2006.03.001
Muddanna MH, Baral SS (2021) Bioleaching of rare earth elements from spent fluid catalytic cracking catalyst using Acidothiobacillus ferrooxidans. J Environ Chem Eng 9:104848. https://doi.org/10.1016/j.jece.2020.104848
Naseri T, Mousavi SM (2022) Insights into the polysaccharides and proteins production from Penicillium citrinum during bioleaching of spent coin cells. Int J Biol Macromol 209:1133–1143. https://doi.org/10.1016/j.ijbiomac.2022.04.042
Natarajan, K.A. (2018) Biotechnology of metals: Principles, recovery methods, and environmental concerns, (1st ed.), Elsevier. 488 pages, https://doi.org/10.1016/C2015-0-00161-7
Niu Z, Huang Q, Xin B, Qi C, Hu J, Chen S, Li Y (2016) Optimization of bioleaching conditions for metal removal from spent zinc-manganese batteries using response surface methodology. J Chem Technol Biotechnol 91:608–617. https://doi.org/10.1002/jctb.4611
Nkulu G, Gaydardzhiev S, Mwema E (2013) Statistical analysis of bioleaching copper, cobalt and nickel from polymetalic concentrate originating from Kamoya deposit in the Democratic Republic of Congo. Miner Eng 48:77–85. https://doi.org/10.1016/j.mineng.2012.10.007
Op de Beeck M, Persson P, Tunlid A (2021) Fungal extracellular polymeric substance matrices – Highly specialized microenvironments that allow fungi to control soil organic matter decomposition reactions. Soil Biol Biochem 159:108304. https://doi.org/10.1016/j.soilbio.2021.108304
Opara CB, Blannin R, Ebert D, Frenzel M, Pollmann K, Kutschke S (2022) Bioleaching of metal(loid)s from sulfidic mine tailings and waste rock from the Neves Corvo mine, Portugal, by an acidophilic consortium. Miner Eng 188:107831. https://doi.org/10.1016/j.mineng.2022.107831
Pathak A, Dastidar MG, Sreekrishnan TR (2009) Bioleaching of heavy metals from sewage sludge by indigenous iron-oxidizing microorganisms using ammonium ferrous sulfate and ferrous sulfate as energy sources: a comparative study. J Hazard Mater 171:273–278. https://doi.org/10.1016/j.jhazmat.2009.05.139
Pathak A, Srichandan H, Kim D-J (2015) Feasibility of bioleaching in removing metals (Al, Ni, V and Mo) from as received raw petroleum spent refinery catalyst: a comparative study on leaching yields risk assessment code and reduced partition index. Mater Trans 56:1278–1286. https://doi.org/10.2320/matertrans.M2015104
Pathak A, Srichandan H, Kim DJ (2019) Column bioleaching of metals from refinery spent catalyst by Acidithiobacillus thiooxidans: effect of operational modifications on metal extraction, metal precipitation, and bacterial attachment. J Environ Manage 242:372–383. https://doi.org/10.1016/j.jenvman.2019.04.081
Potysz A, van Hullebusch ED, Kierczak J (2018) Perspectives regarding the use of metallurgical slags as secondary metal resources – A review of bioleaching approaches. J Environ Manage 219:138–152. https://doi.org/10.1016/j.jenvman.2018.04.083
Potysz A, Mikoda B, Napieraj M (2021) (Bio)dissolution of glassy and diopside-bearing metallurgical slags: experimental and economic aspects. Minerals 11:262. https://doi.org/10.3390/min11030262
Pourhossein F, Mousavi SM, Beolchini F, lo Martire, M. (2021) Novel green hybrid acidic-cyanide bioleaching applied for high recovery of precious and critical metals from spent light emitting diode lamps. J Clean Prod 298:126714. https://doi.org/10.1016/j.jclepro.2021.126714
Qayyum S, Meng K, Pervez S, Nawaz F, Peng C (2019) Optimization of pH, temperature and carbon source for bioleaching of heavy metals by Aspergillus flavus isolated from contaminated soil. Main Group Met Chem 42:1–7. https://doi.org/10.1515/mgmc-2018-0038
Qian C, Wang J, Yan K, Chu H, Tian B, Xin B (2020) Optimization of thermal pre-treatment for simultaneous and efficient release of both Co and Mo from used CoMo catalyst by bioleaching and their mechanisms. Hydrometallurgy 198:105389. https://doi.org/10.1016/j.hydromet.2020.105389
Rasoulnia P, Mousavi SM (2016) Maximization of organic acids production by Aspergillus niger in a bubble column bioreactor for V and Ni recovery enhancement from power plant residual ash in spent-medium bioleaching experiments. Biores Technol 216:729–736. https://doi.org/10.1016/j.biortech.2016.05.114
Rawlings DE, Johnson DB (2007) Biomining, (1st ed.), Springer Berlin, Heidelberg, pp 314, https://doi.org/10.1007/978-3-540-34911-2
Riley AL, MacDonald JM, Burke IT, Renforth P, Jarvis AP, Hudson-Edwards KA, McKie J, Mayes WM (2020) Legacy iron and steel wastes in the UK: extent, resource potential, and management futures. J Geochem Explor 219:106630. https://doi.org/10.1016/j.gexplo.2020.106630
Roberto FF, Schippers A (2022) Progress in bioleaching: part B, applications of microbial processes by the minerals industries. Appl Microbiol Biotechnol 106:5913–5928. https://doi.org/10.1007/s00253-022-12085-9
Rodrigues MLM, Santos GHA, Leôncio HC, Leão VA (2018) Column bioleaching of fluoride-containing secondary copper sulfide ores: experiments with Sulfobacillus thermosulfidooxidans. Front Bioeng Biotechnol 6:183. https://doi.org/10.3389/fbioe.2018.00183
Roy JJ, Cao B, Madhavi S (2021a) A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 282:130944. https://doi.org/10.1016/j.chemosphere.2021.130944
Roy JJ, Madhavi S, Cao B (2021b) Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process. J Clean Prod 280:124242. https://doi.org/10.1016/j.jclepro.2020.124242
Sadhukhan J, Ng KS, Hernandez EM (2014) Biorefineries and chemical processes: design integration and sustainability analysis. John Wiley & Sons Ltd, Chichester, UK. https://doi.org/10.1002/9781118698129
Sand W, Gehrke T, Jozsa PG, Schippers A (2001) (Bio)chemistry of bacterial leaching - direct vs. indirect bioleaching. Hydrometallurgy 59:159–175. https://doi.org/10.1016/S0304-386X(00)00180-8
Sarker SK, Haque N, Bhuiyan M, Bruckard W, Pramanik BK (2022) Recovery of strategically important critical minerals from mine tailings. J Environ Chem Eng 10:107622. https://doi.org/10.1016/j.jece.2022.107622
Shahbaz A (2022) A systematic review on leaching of rare earth metals from primary and secondary sources. Miner Eng 184:107632. https://doi.org/10.1016/j.mineng.2022.107632
Soto PE, Galleguillos PA, Serón MA, Zepeda VJ, Demergasso CS, Pinilla C (2013) Parameters influencing the microbial oxidation activity in the industrial bioleaching heap at Escondida mine, Chile. Hydrometallurgy 133:51–57. https://doi.org/10.1016/j.hydromet.2012.11.011
Srichandan H, Mohapatra RK, Parhi PK, Mishra S (2019) Bioleaching approach for extraction of metal values from secondary solid wastes: a critical review. Hydrometallurgy 189:105122. https://doi.org/10.1016/j.hydromet.2019.105122
Srichandan H, Mohapatra RK, Singh PK, Mishra S, Parhi PK, Naik K (2020) Column bioleaching applications, process development, mechanism, parametric effect and modelling: a review. J Ind Eng Chem 90:1–16. https://doi.org/10.1016/j.jiec.2020.07.012
Sun M, Wang Y, Hong J, Dai J, Wang R, Niu Z, Xin B (2016) Life cycle assessment of a bio-hydrometallurgical treatment of spent Zn-Mn batteries. J Clean Prod 129:350–358. https://doi.org/10.1016/j.jclepro.2016.04.058
Sur IM, Micle V, Gabor T (2018) Heavy metals removal by bioleaching using Thiobacillus ferrooxidans. Romanian Biotechnol Lett 23:13409
Tezyapar Kara I, Marsay N, Huntington V, Coulon F, Alamar MC, Capstick M, Higson S, Buchanan A, Wagland S (2022) Assessing metal recovery opportunities through bioleaching from past metallurgical sites and waste deposits: UK case study. Detritus 21:62–71. https://doi.org/10.31025/2611-4135/2022.17232
Thompson VS, Gupta M, Jin H, Vahidi E, Yim M, Jindra MA, Nguyen V, Fujita Y, Sutherland JW, Jiao Y, Reed DW (2018) Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain Chem Eng 6:1602–1609. https://doi.org/10.1021/acssuschemeng.7b02771
Tian B, Cui Y, Qin Z, Wen L, Li Z, Chu H, Xin B (2022) Indirect bioleaching recovery of valuable metals from electroplating sludge and optimization of various parameters using response surface methodology (RSM). J Environ Manag 312:114927. https://doi.org/10.1016/j.jenvman.2022.114927
Torma AE, Itzkovitch IJ (1976) Influence of organic solvents on chalcopyrite oxidation ability of Thiobacillus ferrooxidans. Appl Environ Microbiol 32:102–107. https://doi.org/10.1128/aem.32.1.102-107.1976
Trivedi A, Vishwakarma A, Saawarn B, Mahanty B, Hait S (2022) Fungal biotechnology for urban mining of metals from waste printed circuit boards: a review. J Environ Manag 323:116133. https://doi.org/10.1016/j.jenvman.2022.116133
Vargas T, Estay H, Arancibia E, Díaz-Quezada S (2020) In situ recovery of copper sulfide ores: alternative process schemes for bioleaching application. Hydrometallurgy 196:105442. https://doi.org/10.1016/j.hydromet.2020.105442
Villares M, Işıldar A, Mendoza Beltran A, Guinee J (2016) Applying an ex-ante life cycle perspective to metal recovery from e-waste using bioleaching. J Clean Prod 129:315–328. https://doi.org/10.1016/j.jclepro.2016.04.066
Wang J, Bai J, Xu J, Liang B (2009a) Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J Hazard Mater 172:1100–1105. https://doi.org/10.1016/j.jhazmat.2009.07.102
Wang Q, Yang J, Wang Q, Wu T (2009b) Effects of water-washing pretreatment on bioleaching of heavy metals from municipal solid waste incinerator fly ash. J Hazard Mater 162:812–818. https://doi.org/10.1016/j.jhazmat.2008.05.125
Wang J, Huang Q, Li T, Xin B, Chen S, Guo X, Liu C, Li Y (2015) Bioleaching mechanism of Zn, Pb, In, Ag, Cd and As from Pb/Zn smelting slag by autotrophic bacteria. J Environ Manage 159:11–17. https://doi.org/10.1016/j.jenvman.2015.05.013
Wang S, Zheng Y, Yan W, Chen L, Dummi Mahadevan G, Zhao F (2016) Enhanced bioleaching efficiency of metals from E-wastes driven by biochar. J Hazard Mater 320:393–400. https://doi.org/10.1016/j.jhazmat.2016.08.054
Wang J, Tian B, Bao Y, Qian C, Yang Y, Niu T, Xin B (2018) Functional exploration of extracellular polymeric substances (EPS) in the bioleaching of obsolete electric vehicle LiNixCoyMn1-x-yO2 Li-ion batteries. J Hazard Mater 354:250–257. https://doi.org/10.1016/j.jhazmat.2018.05.009
Watling HR (2014) Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals 5:1–60. https://doi.org/10.3390/min5010001
Watling HR, Collinson DM, Fjastad S, Kaksonen AH, Li J, Morris C, Perrot FA, Rea SM, Shiers DW (2014) Column bioleaching of a polymetallic ore: effects of pH and temperature on metal extraction and microbial community structure. Miner Eng 58:90–99. https://doi.org/10.1016/j.mineng.2014.01.022
Whitworth AJ, Forbes E, Verster I, Jokovic V, Awatey B, Parbhakar-Fox A (2022) Review on advances in mineral processing technologies suitable for critical metal recovery from mining and processing wastes. Clean Eng Technol 7:100451. https://doi.org/10.1016/j.clet.2022.100451
Xu TJ, Ting YP (2004) Optimisation on bioleaching of incinerator fly ash by Aspergillus niger - Use of central composite design. Enzyme Microb Technol 35:444–454. https://doi.org/10.1016/j.enzmictec.2004.07.003
Yaashikaa PR, Priyanka B, Senthil Kumar P, Karishma S, Jeevanantham S, Indraganti S (2022) A review on recent advancements in recovery of valuable and toxic metals from e-waste using bioleaching approach. Chemosphere 287:132230. https://doi.org/10.1016/j.chemosphere.2021.132230
Yang Y, Diao M, Liu K, Qian L, Nguyen A, v. and Qiu, G. (2013) Column bioleaching of low-grade copper ore by Acidithiobacillus ferrooxidans in pure and mixed cultures with a heterotrophic acidophile Acidiphilium sp. Hydrometallurgy 131–132:93–98. https://doi.org/10.1016/j.hydromet.2012.09.003
Yi Q, Wu S, Southam G, Robertson L, You F, Liu Y, Wang S, Saha N, Webb R, Wykes J, Chan TS, Lu YR, Huang L (2021) Acidophilic iron- and sulfur-oxidizing bacteria, Acidithiobacillus ferrooxidans, drives alkaline pH neutralization and mineral weathering in Fe ore tailings. Environ Sci Technol 55:8020–8034. https://doi.org/10.1021/acs.est.1c00848
Yin S, Wang L, Kabwe E, Chen X, Yan R, An K, Zhang L, Wu A (2018) Copper bioleaching in China: review and prospect. Minerals 8:32. https://doi.org/10.3390/min8020032
Zanbak C (2012) Heap leaching technique in mining within the context of best available techniques (BAT), 36 pp. http://www.euromines.org/files/mining-europe/mining-techniques/batforheapleaching-feb2013-c.zanbak-euromines.pdf
Zare Tavakoli H, Abdollahy M, Ahmadi SJ, Khodadadi Darban A (2017a) The effect of particle size, irrigation rate and aeration rate on column bioleaching of uranium ore. Russian J Non-Ferrous Met 58:188–199. https://doi.org/10.3103/S106782121703018X
Zare Tavakoli H, Abdollahy M, Ahmadi SJ, Darban AK (2017b) Enhancing recovery of uranium column bioleaching by process optimization and kinetic modeling. Trans Nonferrous Met Soc China (english Edition) 27:2691–2703. https://doi.org/10.1016/S1003-6326(17)60298-X
Zare Tavakoli H, Abdollahy M, Ahmadi SJ, Khodadadi Darban A (2017c) Kinetics of uranium bioleaching in stirred and column reactors. Miner Eng 111:36–46. https://doi.org/10.1016/j.mineng.2017.06.003
Zeng G, Luo S, Deng X, Li L, Au C (2013) Influence of silver ions on bioleaching of cobalt from spent lithium batteries. Miner Eng 49:40–44. https://doi.org/10.1016/j.mineng.2013.04.021
Zhang L, Zhou W, Liu Y, Jia H, Zhou J, Wei P, Zhou H (2020) Bioleaching of dewatered electroplating sludge for the extraction of base metals using an adapted microbial consortium: process optimization and kinetics. Hydrometallurgy 191:105227. https://doi.org/10.1016/j.hydromet.2019.105227
Acknowledgements
This research was funded by the European Regional Development Fund as part of the Interreg Northwest Europe project “Regeneration of past metallurgical sites and deposits through innovative circularity for raw materials” (REGENERATIS) (NWE918). The authors would also like to thank Republic of Turkey Ministry of National Education, Study Abroad Program, for sponsoring I. Tezyapar Kara.
Funding
This research was funded by the European Regional Development Fund as part of the Interreg Northwest Europe project “Regeneration of past metallurgical sites and deposits through innovative circularity for raw materials” (REGENERATIS) (NWE918), and the Republic of Turkey Ministry of National Education, Study Abroad Program.
Author information
Authors and Affiliations
Contributions
Conceptualisation, I.T.K and F.C.; Writing, original draft preparation, I.T.K; Writing, review and editing, F.C., S.T.W. and K.K.; Supervision and feedback, F.C. and S.T.W.; project administration, F.C., Funding acquisition, F.C. and S.T.W. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following conflict of interests: Stuart T. Wagland is an associate editor for Environmental Chemistry Letters.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tezyapar Kara, I., Kremser, K., Wagland, S.T. et al. Bioleaching metal-bearing wastes and by-products for resource recovery: a review. Environ Chem Lett 21, 3329–3350 (2023). https://doi.org/10.1007/s10311-023-01611-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01611-4