Abstract
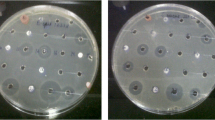
Marine-derived actinobacteria have tremendous potential to produce novel metabolites with diverse biological activities. The Andaman coast of India has a lot of microbial diversity, but it is still a relatively unknown ecology for isolating novel actinobacteria with beneficial bioactive compounds. We have isolated 568 actinobacterial strains from mangrove rhizosphere sediments and sponge samples. Crude extracts from 75 distinct strains were produced by agar surface fermentation and extracted using ethyl acetate. In the disc diffusion method, 25 actinobacterial strains showed antimicrobial activity; notably, the strain MAB56 demonstrated promising broad-spectrum activity. Strain MAB56 was identified as Streptomyces albus by cultural, microscopic, and molecular methods. Conditions for bioactive metabolites from MAB56 were optimized and produced in a lab-scale fermenter. Three active metabolites (C1, C2, and C3) that showed promising broad-spectrum antimicrobial activity were isolated through HPLC-based purification. Based on the UV, FT-IR, NMR, and LC–MS analysis, the chemical nature of the active compounds was confirmed as 12-methyltetradecanoic acid (C1), palmitic acid (C2), and tridecanoic acid (C3) with molecular formulae C14H28O2, C16H32O2, and C13H26O2, respectively. Interestingly, palmitic acid (C2) also exhibited anti-HIV activity with an IC50 value of < 1 µg/ml. Our findings reveal that the actinobacteria from the Andaman marine ecosystems are promising for isolating anti-infective metabolites.




Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Baptista, P. V., McCusker, M. P., Carvalho, A., Ferreira, D. A., Mohan, N. M., Martins, M., & Fernandes, A. R. (2018). Nano-strategies to fight multidrug resistant bacteria-“A Battle of the Titans.” Frontiers in Microbiology, 9, 1441.
Jose, P. A., Maharshi, A., & Jha, B. (2021). Actinobacteria in natural products research: Progress and prospects. Microbiological Research, 246, 126708.
Genilloud, O. (2017). Actinomycetes: Still a source of novel antibiotics. Natural Product Reports, 34(10), 1203–1232.
Berdy, J. (2012). Thoughts and facts about antibiotics: Where we are now and where we are heading. The Journal of Antibiotics, 65(8), 385–395.
Lam, K. S. (2006). Discovery of novel metabolites from marine actinomycetes. Current Opinion in Microbiology, 9(3), 245–251.
Raveh, A., Delekta, P. C., Dobry, C. J., Peng, W., Schultz, P. J., Blakely, P. K., Tai, A. W., Matainaho, T., Irani, D. N., Sherman, D. H., & Miller, D. J. (2013). Discovery of potent broad spectrum antivirals derived from marine actinobacteria. PLoS ONE, 8(12), e82318.
Valliappan, K., Sun, W., & Li, Z. (2014). Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Applied Microbiology and Biotechnology, 98(17), 7365–7377.
Goel, N., Ahmad, R., Singh, R., Sood, S., & Khare, S. K. (2021). Biologically synthesized silver nanoparticles by Streptomyces sp. EMB24 extracts used against the drug-resistant bacteria. Bioresource Technology Reports, 5, 100753.
Dhakal, D., Pokhrel, A. R., Shrestha, B., & Sohng, J. K. (2017). Marine rare actinobacteria: Isolation, characterization, and strategies for harnessing bioactive compounds. Frontiers in Microbiology, 8, 1106.
Blunt, J. W., Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., & Prinsep, M. R. (2018). Marine natural products. . Natural Product Reports, 35(1), 8–53.
Sarkar, S., Saha, M., Roy, D., Jaisankar, P., Das, S., Gauri Roy, L., Gachhui, R., Sen, T., & Mukherjee, J. (2008). Enhanced production of antimicrobial compounds by three salt-tolerant actinobacterial strains isolated from the Sundarbans in a niche-mimic bioreactor. Marine Biotechnology (New York. N.Y.), 10(5), 518–526.
Saha, M., Jaisankar, P., Das, S., Sarkar, K. K., Roy, S., Besra, S. E., Vedasiromani, J. R., Ghosh, D., Sana, B., & Mukherjee, J. (2006). Production and purification of a bioactive substance inhibiting multiple drug resistant bacteria and human leukemia cells from a salt-tolerant marine Actinobacterium sp isolated from the Bay of Bengal. Biotechnology Letters, 28(14), 1083–1088.
Mincer, T. J., Jensen, P. R., Kauffman, C. A., & Fenical, W. (2002). Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Applied and Anvironmental Microbiology, 68(10), 5005–5011.
Zhang, H., Lee, Y. K., Zhang, W., & Lee, H. K. (2006). Culturable actinobacteria from the marine sponge Hymeniacidon perleve: Isolation and phylogenetic diversity by 16S rRNA gene-RFLP analysis. Antonie van Leeuwenhoek, 90(2), 159–169.
Radhakrishnan, M., Gopikrishnan, V., Balaji, S., Balagurunathan, R., & Kumar, V. (2014). Bioprospecting of actinomycetes from certain less explored ecosystems active against Mycobacterium tuberculosis and other non-mycobacterial pathogens. International Scholarly Research Notices, 2014, 812974.
Shirling, E. B., & Gottileb, D. (1966). Methods for characterization of Streptomyces species. International Journal of Systematic and Evolutionary Microbiology, 16, 313–340.
Hasegawa, T., Takizawa, M., & Tanida, S. (1983). A rapid analysis for chemical grouping of aerobic actinomycetes. Journal of General and Applied Microbiology, 29, 319–322.
Gopikrishnan, V., Radhakrishnan, M., Shanmugasundaram, T., Ramakodi, M. P., & Balagurunathan, R. (2019). Isolation, characterization and identification of antibiofouling metabolite from mangrove derived Streptomyces sampsonii PM33. Scientific Reports, 9(1), 12975.
Manigundan, K., Joseph, J., Radhakrishnan, M., Sivarajan, A., & Balagurunathan, R. (2021). Multifaceted bioproperties of Streptomyces bacillaris ANS2 isolated from Andaman and Nicobar Islands. India. Research Journal of Biotechnology, 16(11), 99–108.
Wiegand, I., Hilpert, K., & Hancock, R. E. (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols, 3(2), 163–175.
Vaishnavi, M., Manigundan, K., Smalia, T., Gopikrishnan, V., Kumar, A., Hanna, L. E., Ushanandhini, P., Krupakar, P., & Radhakrishnan, M. (2020). Antimicrobial and anti-HIV activity of extracellular pigment from Streptomyces sp. S45 isolated from forest soil, Sabarimala forest, Kerala. India. Indian Journal of Experimental Biology, 58, 861–868.
Cragg, G. M., & Newman, D. J. (2013). Natural products: A continuing source of novel drug leads. Biochimica et Biophysica Acta, 1830(6), 3670–3695.
Jagannathan, S. V., Manemann, E. M., Rowe, S. E., Callender, M. C., & Soto, W. (2021). Marine actinomycetes, new sources of biotechnological products. Marine Drugs, 19(7), 365.
Meena, B., Anburajan, L., & Johnthini, M. A. (2022). Exploration of mangrove-associated actinobacteria from South Andaman Islands. Systems Microbiology and Biomanufacturing. https://doi.org/10.1007/s43393-022-00134-3
Pavan Kumar, J. G. S., Gomathi, A., Gothandam, K. M., & Vasconcelos, V. (2018). Bioactivity assessment of Indian origin-mangrove actinobacteria against Candida albicans. Marine Drugs, 16(2), 60.
Kavitha, A., & Savithri, H. S. (2017). Biological significance of marine actinobacteria of East Coast of Andhra Pradesh. India. Frontiers in Microbiology, 8, 1201.
Sengupta, S., Pramanik, A., Ghosh, A., & Bhattacharyya, M. (2015). Antimicrobial activities of actinomycetes isolated from unexplored regions of Sundarbans mangrove ecosystem. BMC Microbiology, 15, 170.
Ser, H. L., Tan, L. T., Palanisamy, U. D., AbdMalek, S. N., Yin, W. F., Chan, K. G., Goh, B. H., & Lee, L. H. (2016). Streptomyces antioxidans sp. nov. a novel mangrove soil actinobacterium with antioxidative and neuroprotective potentials. Frontiers in Microbiology, 7, 899.
Tan, L. T., Ser, H. L., Yin, W. F., Chan, K. G., Lee, L. H., & Goh, B. H. (2015). Investigation of antioxidative and anticancer potentials of Streptomyces sp MUM256 isolated from Malaysia mangrove soil. Frontiers in Microbiology, 6, 1316.
Andreote, F. D., Jiménez, D. J., Chaves, D., Dias, A. C., Luvizotto, D. M., Dini-Andreote, F., Fasanella, C. C., Lopez, M. V., Baena, S., Taketani, R. G., & de Melo, I. S. (2012). The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLoS ONE, 7(6), e38600.
Imchen, M., Kumavath, R., Barh, D., Azevedo, V., Ghosh, P., Viana, M., & Wattam, A. R. (2017). Searching for signatures across microbial communities: Metagenomic analysis of soil samples from mangrove and other ecosystems. Scientific Reports, 7(1), 8859.
Lee, L. H., Zainal, N., Azman, A. S., Eng, S. K., Goh, B. H., Yin, W. F., Ab Mutalib, N. S., & Chan, K. G. (2014). Diversity and antimicrobial activities of actinobacteria isolated from tropical mangrove sediments in Malaysia. The Scientific World Journal, 2014, 698178.
Chakraborty, B., Kumar, R. S., Almansour, A. I., Gunasekaran, P., & Nayaka, S. (2022). Bioprospection and secondary metabolites profiling of marine Streptomyces levis strain KS46. Saudi Journal of Biological Sciences, 29(2), 667–679.
Fahmy, N. M., & Abdel-Tawab, A. M. (2021). Isolation and characterization of marine sponge-associated Streptomyces sp. NMF6 strain producing secondary metabolite(s) possessing antimicrobial, antioxidant, anticancer, and antiviral activities. Journal, Genetic Engineering & Biotechnology, 19(1), 102.
Ramalingam, V., Rajaram, R., Archunan, G., Padmanabhan, P., & Gulyás, B. (2022). Structural characterization, antimicrobial, antibiofilm, antioxidant, anticancer and acute toxicity properties of N-(2-hydroxyphenyl)-2-phenazinamine from Nocardiopsis exhalans (KP149558). Frontiers in Cellular and Infection Microbiology, 12, 794338.
Xu, D., Han, L., Li, C., Cao, Q., Zhu, D., Barrett, N. H., Harmody, D., Chen, J., Zhu, H., McCarthy, P. J., Sun, X., & Wang, G. (2018). Bioprospecting deep-sea actinobacteria for novel anti-infective natural products. Frontiers in Microbiology, 9, 787.
Mothana, A. A., Al-Shamahy, H. A., Mothana, R. A., Khaled, J. M., Al-Rehaily, A. J., Al-Mahdi, A. Y., & Lindequist, U. (2022). Streptomyces sp. 1S1 isolated from Southern coast of the Red Sea as a renewable natural resource of several bioactive compounds. Saudi pharmaceutical journal: SPJ: the official publication of the Saudi Pharmaceutical Society, 30(2), 162–171.
Acknowledgements
The authors thank the authorities of Annamalai University, Periyar University, and Sathyabama Institute of Science and Technology for the research facilities provided. The authors also acknowledge the Department of Biotechnology, New Delhi (BT/PR5426/AAQ/3/599/2012; BT/PR41474/NDB/39/760/2020 dated 28.09.2021) for their support in the form of research grant
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manikkam, R., Murthy, S., Palaniappan, S. et al. Antibacterial and Anti-HIV Metabolites from Marine Streptomyces albus MAB56 Isolated from Andaman and Nicobar Islands, India. Appl Biochem Biotechnol 195, 7738–7754 (2023). https://doi.org/10.1007/s12010-023-04493-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04493-y