Abstract
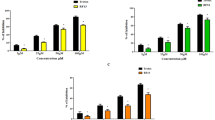
Obesity is linked to the development of major metabolic disorders such as type 2 diabetes, cardiovascular disease, and cancer. Recent research has focused on the molecular link between obesity and oxidative stress. Obesity impairs antioxidant function, resulting in dramatically increased reactive oxygen levels and apoptosis. In this study, we investigated the effect of IW13 peptide on inhibiting lipid accumulation and regulating the antioxidant mechanism to normalize the lipid metabolism in HFD induced zebrafish larvae. Our results showed that co-treatment with IW13 peptide showed a protective effect in HFD zebra fish larvae by increasing the survival and heart rate. However, IW13 peptide co-treatment reduced triglycerides and cholesterol levels while also restoring the SOD and CAT antioxidant enzymes. In addition, IW13 co-treatment inhibited the formation of lipid peroxidation and superoxide anion by regulating the glutathione level. Also, the results showed that IW13 specifically downregulated the expression of the lipogenic-specific genes (C/EBP-α, SREBP1, and FAS). The findings exhibited that the IW13 peptide with effective antioxidant and anti-obesity activity could act as a futuristic drug to treat obesity and oxidative stress-related diseases.
Graphical Abstract







Similar content being viewed by others
Data Availability
Data is available on request from the authors.
References
Perez-Escamilla, R., Obbagy, J. E., Altman, J. M., Essery, E. V., McGrane, M. M., Wong, Y. P., & Williams, C. L. (2012). Dietary energy density and body weight in adults and children: A systematic review. Journal of the Academy of Nutrition and Dietetics, 112, 671–684. https://doi.org/10.1016/j.jand.2012.01.020
Savini, I., Catani, M. V., Evangelista, D., Gasperi, V., & Avigliano, L. (2013). Obesity-associated oxidative stress: Strategies finalized to improve redox state. International Journal of Molecular Sciences, 14, 10497–10538. https://doi.org/10.3390/ijms140510497
Poitout, V., Hagman, D., Stein, R., Artner, I., Robertson, R. P., & Harmon, J. S. (2006). Recent advances in nutritional sciences. The Journal of Nutrition, 136, 873–876.
Serra, D., Mera, P., Malandrino, M. I., Mir, J. F., & Herrero, L. (2013). Mitochondrial fatty acid oxidation in obesity. Antioxidants and Redox Signaling, 19, 269–284. https://doi.org/10.1089/ars.2012.4875
Guru, A., Sudhakaran, G., Velayutham, M., Murugan, R., Pachaiappan, R., Mothana, R. A., & Arockiaraj, J. (2022). Daidzein normalized gentamicin-induced nephrotoxicity and associated pro-inflammatory cytokines in MDCK and zebrafish: Possible mechanism of nephroprotection. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 258, 109364. https://doi.org/10.1016/j.cbpc.2022.109364
Haridevamuthu, B., Manjunathan, T., Guru, A., Alphonse, R. W., & C., Boopathi, S., Murugan, R., & Arockiaraj, J. (2022). Amelioration of acrylamide induced neurotoxicity by benzo[b]thiophene analogs via glutathione redox dynamics in zebrafish larvae. Brain Research, 1788, 147941. https://doi.org/10.1016/j.brainres.2022.147941
Suleiman, J. B., Nna, V. U., Zakaria, Z., Othman, Z. A., Bakar, A. B. A., & Mohamed, M. (2020). Obesity-induced testicular oxidative stress, inflammation and apoptosis: Protective and therapeutic effects of orlistat. Reproductive Toxicology, 95, 113–122. https://doi.org/10.1016/j.reprotox.2020.05.009
Bryan, S., Baregzay, B., Spicer, D., Singal, P. K., & Khaper, N. (2013). Redox-inflammatory synergy in the metabolic syndrome. Canadian Journal of Physiology and Pharmacology, 91, 22–30. https://doi.org/10.1139/cjpp-2012-0295
Guru, A., Velayutham, M., & Arockiaraj, J. (2022). Lipid-lowering and antioxidant activity of RF13 peptide from vacuolar protein sorting-associated protein 26B (VPS26B) by modulating lipid metabolism and oxidative stress in HFD induced obesity in zebrafish larvae. International Journal of Peptide Research and Therapeutics, 28, 74. https://doi.org/10.1007/s10989-022-10376-3
Thurairajah, P. H., Syn, W. K., Neil, D. A. H., Stell, D., & Haydon, G. (2005). Orlistat (Xenical)-induced subacute liver failure. European Journal of Gastroenterology and Hepatology, 17, 1437–1438. https://doi.org/10.1097/01.meg.0000187680.53389.88
Tziomalos, K., Krassas, G. E., & Tzotzas, T. (2009). The use of sibutramine in the management of obesity and related disorders: An update. Vascular Health and Risk Management, 5, 441–452. https://doi.org/10.2147/vhrm.s4027
Bray, G. A. (2005). Drug treatment of obesity. Psychiatric Clinics of North America, 28, 193–217. https://doi.org/10.1016/j.psc.2004.09.009
Mudgil, P., Kamal, H., Yuen, G. C., & Maqsood, S. (2018). Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chemistry, 259, 46–54. https://doi.org/10.1016/j.foodchem.2018.03.082
Fan, X., Cui, Y., Zhang, R., & Zhang, X. (2018). Purification and identification of anti-obesity peptides derived from Spirulina platensis. Journal of Functional Foods, 47, 350–360. https://doi.org/10.1016/j.jff.2018.05.066
Guru, A., Lite, C., Freddy, A. J., Kumar, P., Pasupuleti, M., Saraswathi, T., & Arshad, A. (2021). Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Developmental and Comparative Immunology, 114, 103863. https://doi.org/10.1016/j.dci.2020.103863
Prabha, N., Guru, A., Harikrishnan, R., Gatasheh, M. K., Hatamleh, A. A., Juliet, A., & Arockiaraj, J. (2022). Neuroprotective and antioxidant capability of RW20 peptide from histone acetyltransferases caused by oxidative stress-induced neurotoxicity in in vivo zebrafish larval model. Journal of King Saud University - Science, 34, 101861. https://doi.org/10.1016/j.jksus.2022.101861
Issac, P. K., Lite, C., Guru, A., Velayutham, M., Kuppusamy, G., Saraswathi, N. T., & Arockiaraj, J. (2021). Tryptophan-tagged peptide from serine threonine-protein kinase of Channa striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3–dependent apoptotic response in zebrafish larvae. Fish Physiology and Biochemistry, 47, 293–311. https://doi.org/10.1007/s10695-020-00912-7
Velayutham, M., Guru, A., Gatasheh, M. K., Hatamleh, A. A., Juliet, A., & Arockiaraj, J. (2022). Molecular docking of SA11, RF13 and DI14 peptides from vacuolar protein sorting associated protein 26B against cancer proteins and in vitro investigation of its anticancer potency in Hep-2 cells. International Journal of Peptide Research and Therapeutics, 28. https://doi.org/10.1007/s10989-022-10395-0
Singh, M., Guru, A., Sudhakaran, G., Pachaiappan, R., Mahboob, S., Juliet, A., et al. (2022). Copper sulfate induced toxicological impact on in-vivo zebrafish larval model protected due to acacetin via anti-inflammatory and glutathione redox mechanism. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 262, 109463. https://doi.org/10.1016/j.cbpc.2022.109463
Haridevamuthu, B., Guru, A., Murugan, R., Sudhakaran, G., Pachaiappan, R., Almutairi, M. H., & Arockiaraj, J. (2022). Neuroprotective effect of Biochanin a against Bisphenol A-induced prenatal neurotoxicity in zebrafish by modulating oxidative stress and locomotory defects. Neuroscience Letters, 790, 136889. https://doi.org/10.1016/j.neulet.2022.136889
Manjunathan, T., Guru, A., Arokiaraj, J., & Gopinath, P. (2021). 6-Gingerol and semisynthetic 6-gingerdione counteract oxidative stress induced by ROS in zebrafish. Chemistry and Biodiversity, 18. https://doi.org/10.1002/cbdv.202100650
Sudhakaran, G., Prathap, P., Guru, A., Haridevamuthu, B., Murugan, R., Almutairi, B. O., & Arockiaraj, J. (2022). Reverse pharmacology of Nimbin-N2 attenuates alcoholic liver injury and promotes the hepatoprotective dual role of improving lipid metabolism and downregulating the levels of inflammatory cytokines in zebrafish larval model. Molecular and Cellular Biochemistry, 477, 2387–2401. https://doi.org/10.1007/s11010-022-04448-7
Guru, A., & Arockiaraj, J. (2022). Exposure to environmental pollutant bisphenol A causes oxidative damage and lipid accumulation in Zebrafish larvae: Protective role of WL15 peptide derived from cysteine and glycine-rich protein 2. Journal of Biochemical and Molecular Toxicology, 37, e23223. https://doi.org/10.1002/jbt.23223
Guru, A., Issac, P. K., Saraswathi, N. T., Seshadri, V. D., Gabr, G. A., & Arockiaraj, J. (2021). Deteriorating insulin resistance due to WL15 peptide from cysteine and glycine-rich protein 2 in high glucose-induced rat skeletal muscle L6 cells. Cell Biology International, 45, 1698–1709. https://doi.org/10.1002/cbin.11608
Velayutham, M., Ojha, B., Issac, P. K., Lite, C., Guru, A., Pasupuleti, M., & Arockiaraj, J. (2021). NV14 from serine O-acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H2O2 and other responses in vivo zebrafish larval model. Cell Biology International, 45, 2331–2346. https://doi.org/10.1002/cbin.11680
Issac, P. K., Guru, A., Velayutham, M., Pachaiappan, R., Arasu, M. V., Al-Dhabi, N. A., & Arockiaraj, J. (2021). Oxidative stress induced antioxidant and neurotoxicity demonstrated in vivo zebrafish embryo or larval model and their normalization due to morin showing therapeutic implications. Life Sciences, 283, 119864. https://doi.org/10.1016/j.lfs.2021.119864
Sudhakaran, G., Rajesh, R., Guru, A., Haridevamuthu, B., & Murugan, R. (2022). Deacetylated nimbin analog N2 fortifies alloxan-induced pancreatic β-cell damage in insulin-resistant zebrafish larvae by upregulating phosphoenolpyruvate carboxykinase (PEPCK) and insulin levels. Toxicology and Applied Pharmacology, 454, 116229. https://doi.org/10.1016/j.taap.2022.116229
Issac, P. K., Karan, R., Guru, A., Pachaiappan, R., Arasu, M. V., Al-Dhabi, N. A., & Raj, J. A. (2021). Insulin signaling pathway assessment by enhancing antioxidant activity due to morin using in vitro rat skeletal muscle L6 myotubes cells. Molecular Biology Reports, 48, 5857–5872. https://doi.org/10.1007/s11033-021-06580-x
Sarkar, P., Guru, A., Raju, S. V., Farasani, A., Oyouni, A. A. A., Alzahrani, O. R., & Arockiaraj, J. (2021). GP13, an Arthrospira platensis cysteine desulfurase-derived peptide, suppresses oxidative stress and reduces apoptosis in human leucocytes and zebrafish (Danio rerio) embryo via attenuated caspase-3 expression. Journal of King Saud University - Science, 33, 101665. https://doi.org/10.1016/j.jksus.2021.101665
Haridevamuthu, B., Manjunathan, T., Guru, A., Kumar, R. S., Rajagopal, R., Kuppusamy, P., & Arockiaraj, J. (2022). Hydroxyl containing benzo[b]thiophene analogs mitigates the acrylamide induced oxidative stress in the zebrafish larvae by stabilizing the glutathione redox cycle. Life Sciences, 298, 120507. https://doi.org/10.1016/j.lfs.2022.120507
Liang, X., Yu, L., Gui, W., & Zhu, G. (2015). Exposure to difenoconazole causes changes of thyroid hormone and gene expression levels in zebrafish larvae. Environmental Toxicology and Pharmacology, 40(3), 983–987. https://doi.org/10.1016/j.etap.2015.10.005
Shieh, Y. S., Chang, Y. S., Hong, J. R., Chen, L. J., Jou, L. K., Hsu, C. C., & Her, G. M. (2010). Increase of hepatic fat accumulation by liver specific expression of Hepatitis B virus X protein in zebrafish. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1801(7), 721–730. https://doi.org/10.1016/j.bbalip.2010.04.008
Cha, S. H., Hwang, Y., Heo, S. J., & Jun, H. S. (2020). Diphlorethohydroxycarmalol attenuates palmitate-induced hepatic lipogenesis and inflammation. Marine Drugs, 18(9), 2–16. https://doi.org/10.3390/md1809047
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25, 402–408. https://doi.org/10.1006/meth.2001.1262
Issac, P. K., Velayutham, M., Guru, A., Sudhakaran, G., Pachaiappan, R., & Arockiaraj, J. (2022). Protective effect of morin by targeting mitochondrial reactive oxygen species induced by hydrogen peroxide demonstrated at a molecular level in MDCK epithelial cells. Molecular Biology Reports, 1–12. https://doi.org/10.1007/s11033-022-07261-z
Huang, C. J., McAllister, M. J., Slusher, A. L., Webb, H. E., Mock, J. T., & Acevedo, E. O. (2015). Obesity-related oxidative stress: The impact of physical activity and diet manipulation. Sports Medicine - Open, 1, 1–12. https://doi.org/10.1186/s40798-015-0031-y
Sudhakaran, G., Guru, A., Muthu, H. D., & B., Murugan, R., Arshad, A., & Arockiaraj, J. (2022). Evidence-based hormonal, mutational, and endocrine-disrupting chemical-induced zebrafish as an alternative model to study PCOS condition similar to mammalian PCOS model. Life Sciences, 291, 120276. https://doi.org/10.1016/j.lfs.2021.120276
Lite, C., Guru, A., Juliet, M. J., & Arockiaraj, J. (2022). Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environmental Toxicology, 37(8), 1988–2004. https://doi.org/10.1002/tox.23545
Manna, P., & Jain, S. K. (2015). Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metabolic Syndrome and Related Disorders, 13, 423–444. https://doi.org/10.1089/met.2015.0095
Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., & Shimomura, I. (2004). Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation, 114, 1752–1761. https://doi.org/10.1172/JCI21625
Ming, G. F., Xiao, D., Gong, W. J., Liu, H. X., Liu, J., Zhou, H. H., & Liu, Z. Q. (2014). JAZF1 can regulate the expression of lipid metabolic genes and inhibit lipid accumulation in adipocytes. Biochemical and Biophysical Research Communications, 445, 673–680. https://doi.org/10.1016/j.bbrc.2014.02.088
Jiang, J. F., Xu, Z. R., Wang, Y. Z., Han, X. Y., & Wang, L. (2006). Postnatal expression pattern of the C/EBP alpha gene in porcine subcutaneous adipose tissue. Journal of Animal and Feed Sciences, 15, 61–70. https://doi.org/10.22358/jafs/66841/2006
Author information
Authors and Affiliations
Contributions
AG: project administration, validation, writing—original draft; GS and SKRN: validation, writing—review and editing; BS and MP: conceptualization, data curation; MM and JA: conceptualization, data curation, supervision, writing—review and editing
Corresponding authors
Ethics declarations
Ethical Approval
In this study, the animal experiment was not applicable.
Consent to Participate
In this study, animals, and human trials are not applicable.
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajay Guru, Gokul Sudhakaran, S. Karthick Raja Namasivayam et al. Serine Threonine-Protein Kinase-Derived IW13 Improves Lipid Metabolism via C/EBP-α/SREBP1/FAS Signaling Pathways in HFD-Induced Zebrafish In Vivo Larval Model. Appl Biochem Biotechnol 195, 4851–4863 (2023). https://doi.org/10.1007/s12010-023-04480-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04480-3