Abstract
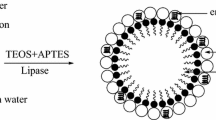
Immobilized lipase is a green and sustainable catalyst for hydrolysis of acidified oil. Glutaraldehyde is widely used for lipase immobilization while the appropriate strategy optimizes the catalytic performance of lipase. In this research, lipase from Candida rugosa (CRL) was immobilized on spherical silica (SiO2) by glutaraldehyde multipoint covalent treatments, including covalent binding method and adsorption-crosslinking method. The enzymatic stability properties and performance in hydrolysis of refined oil and acidified oil were studied. We confirmed that the residual activity decreased while the stability increased because of the influence on secondary structure of lipase after multipoint covalent treatments. In the comparison of different immobilization strategies in multipoint covalent treatment, SiO2-CRL (covalent binding method) showed lower loading capacity than SiO2-CRL (adsorption-crosslinking method), resulting in low activity. However, SiO2-CRL (covalent binding method) showed better reusability and stability. Immobilized lipase via covalent binding method was more potential in the application of catalytic hydrolysis of acidified oils.






Similar content being viewed by others

Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
Ferreira, M. M., Oliveira, G., Basso, R. C., Mendes, A. A., & Hirata, D. B. (2019). Optimization of free fatty acid production by enzymatic hydrolysis of vegetable oils using a non-commercial lipase from Geotrichum candidum. Bioprocess and Biosystems Engineering, 42(10), 1647–1659.
Lima, R. T., Alves, A. M., de Paula, A. V., de Castro, H. F., & Andrade, G. S. S. (2019). Mycelium-bound lipase from Penicillium citrinum as biocatalyst for the hydrolysis of vegetable oils. Biocatalysis and Agricultural Biotechnology, 22, 101410.
Wang, P., Ke, Z. D., Yi, J. J., Liu, X., Hao, L. M., Kang, Q. Z., & Lu, J. K. (2019). Effects of beta-cyclodextrin on the enzymatic hydrolysis of hemp seed oil by lipase Candida sp.99-125. Industrial Crops and Products, 129, 688–693.
Shi, W. Y., Li, T. F., Li, H. B., Du, Q. Y., Zhang, H. X., & Qin, X. H. (2022). Continuous biodiesel production from acidic oil using a combination of the acid-, alkali-catalyzed membrane and GO/PVDF separation membrane. Journal of Industrial and Engineering Chemistry, 107, 268–279.
Jiang, X., Long, F., Zhai, Q. L., Zhao, J. P., Liu, P., & Xu, J. M. (2022). Catalytic cracking of acidified oil and modification of pyrolytic oils from soap stock for the production of a high-quality biofuel. New Journal of Chemistry, 46(4), 1770–1778.
Hamzah, M. A. F., Jahim, J. M., Abdul, P. M., & Asis, A. J. (2019). Investigation of temperature effect on start-up operation from anaerobic digestion of acidified palm oil mill effluent. Energies, 12(13), 2473.
Ma, L. L., Lv, E. M., Du, L. X., Lu, J., & Ding, J. C. (2016). Statistical modeling/optimization and process intensification of microwave-assisted acidified oil esterification. Energy Conversion and Management, 122, 411–418.
Abd Nasir, M. A., Jahim, J. M., Abdul, P. M., Silvamany, H., Maaroff, R. M., & Yunus, M. F. M. (2019). The use of acidified palm oil mill effluent for thermophilic biomethane production by changing the hydraulic retention time in anaerobic sequencing batch reactor. International Journal of Hydrogen Energy, 44(6), 3373–3381.
Barnebey, H. L., & Brown, A. C. (1948). Continuous fat splitting plants using the colgate-emery process. Journal of the American Oil Chemists Society, 25, 95–99.
Urrutia, P., Arrieta, R., Alvarez, L., Cardenas, C., Mesa, M., & Wilson, L. (2018). Immobilization of lipases in hydrophobic chitosan for selective hydrolysis of fish oil: The impact of support functionalization on lipase activity, selectivity and stability. International journal of biological macromolecules, 108, 674–686.
Zied, Z., Edahech, A., Rigano, F., Micalizzi, G., Mondello, L., Kharrat, N., Sellami, M., & Cacciola, F. (2018). Monoacylglycerol and diacylglycerol production by hydrolysis of refined vegetable oil by-products using an immobilized lipase from Serratia sp. W3. Journal of Separation Science, 41(23), 4323–4330.
Mohamad, N. R., Marzuki, N. H. C., Buang, N. A., Huyop, F., & Wahab, R. A. (2015). An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnology and Biotechnological Equipment, 29(2), 205–220.
Butler, M. F., Ng, Y. F., & Pudney, P. D. A. (2003). Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. Journal of Polymer Science Part A: Polymer Chemistry, 41(24), 3941–3953.
Mi, F. L., Shyu, S. S., & Peng, C. K. (2005). Characterization of ring-opening polymerization of genipin and pH-dependent cross-linking reactions between chitosan and genipin. Journal of Polymer Science Part A Polymer Chemistry, 43(10), 1985–2000.
Ren, Y., Abbood, H. A., He, F., Peng, H., & Huang, K. (2013). Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chemical Engineering Journal, 226, 300–311.
Xue, F., Chen, Q., Li, Y., Liu, E., & Li, D. (2019). Immobilized lysozyme onto 1,2,3,4-butanetetracarboxylic (BTCA)-modified magnetic cellulose microsphere for improving bio-catalytic stability and activities. Enzyme and Microbial Technology, 131, 109425.
Pinto, F., Fernandes, F. R., Caldeira, V., Castro, H., Souza, L. D., & Santos, A. (2021). Influence of the procedure to immobilize lipase on SBA-15 for biodiesel production from palm kernel oil. Catalysis Letters, 151, 2187–2196.
Wong, W. K. L., Wahab, R. A., & Onoja, E. (2020). Chemically modified nanoparticles from oil palm ash silica-coated magnetite as support for Candida rugosa lipase-catalysed hydrolysis: Kinetic and thermodynamic studies. Chemical Papers, 74(4), 1253–1265.
Xie, W. L., & Zang, X. Z. (2016). Immobilized lipase on core-shell structured Fe3O4-MCM-41 nanocomposites as a magnetically recyclable biocatalyst for interesterification of soybean oil and lard. Food Chemistry, 194, 1283–1292.
Fahimeh, E., Nematian, T., Salehi, Z., Khodadadi, A. A., & Dalai, A. K. (2021). Amine and aldehyde functionalized mesoporous silica on magnetic nanoparticles for enhanced lipase immobilization, biodiesel production, and facile separation. Fuel, 291(3), 120126.
Yu, D., Zhang, X., Wang, T., Geng, H., & Elfalleh, W. (2021). Immobilized Candida antarctica lipase B (CALB) on functionalized MCM-41: Stability and catalysis of transesterification of soybean oil and phytosterol. Food Bioscience, 40, 100906.
Serpa, J. D., Matias, G. A. B., Fechine, P. B. A., da Costa, V. M., Freire, R. M., Denardin, J. C., Goncalves, L. R. B., de Macedo, A. C., & Rocha, M. V. P. (2021). New nanocomposite made of cashew apple bagasse lignin and Fe3O4 for immobilizing of lipase B from Candida antarctica aiming at esterification. Journal of Chemical Technology and Biotechnology, 96(9), 2472–2487.
Gao, S. L., Wang, Y. J., Diao, X., Luo, G. S., & Dai, Y. Y. (2010). Effect of pore diameter and cross-linking method on the immobilization efficiency of Candida rugosa lipase in SBA-15. Bioresource Technology, 101(11), 3830–3837.
Kim, M. I., Kim, J., Lee, J., Jia, H., Na, H. B., Youn, J. K., Kwak, J. H., Dohnalkova, A., Grate, J. W., & Wang, P. (2010). Crosslinked enzyme aggregates in hierarchically-ordered mesoporous silica: A simple and effective method for enzyme stabilization. Biotechnology and Bioengineering, 96(2), 210–218.
Alamsyah, G., Albels, V. A., Sahlan, M., & Hermansyah, H. (2017). Effect of chitosan’s amino group in adsorption-crosslinking immobilization of lipase enzyme on resin to catalyze biodiesel synthesis. Energy Procedia, 136, 47–52.
Rathankumar, A. K., Sailavanyaa, S., Saikia, K., Gururajan, A., Sivanesan, S., Gosselin, M., Vaidyanathan, V. K., & Cabana, H. (2019). Systemic concocting of crosslinked enzyme aggregates of Candida antarctica lipase B (Novozyme 435) for the biomanufacturing of rhamnolipids. Journal of Surfactants and Detergents, 22(3), 477–490.
Nuraliyah, A., Perdani, M. S., Putri, D. N., Sahlan, M., Wijanarko, A., & Hermansyah, H. (2021). Effect of additional amino group to improve the performance of immobilized lipase from Aspergillus niger by adsorption-crosslinking method. Frontiers in Energy Research, 9, 616945.
Macquarrie, D. J., Jackson, D. B., Tailland, S., & Utting, K. A. (2001). Organically modified hexagonal mesoporous silicas (HMS)-remarkable effect of preparation solvent on physical and chemical properties. Journal of Materials Chemistry, 11(7), 1843–1849.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Gupta, N., Rathi, P., & Gupta, R. (2002). Simplified para-nitrophenyl palmitate assay for lipases and esterases. Analytical Biochemistry, 311, 98–99.
Cai, Z. X., Wei, Y., Wu, M., Guo, Y. L., Xie, Y. P., Tao, R., Li, R. Q., Wang, P. G., Ma, A. Q., & Zhang, H. B. (2019). Lipase immobilized on layer-by-layer polysaccharide-coated Fe3O4@SiO2 microspheres as a reusable biocatalyst for the production of structured lipids. Acs Sustainable Chemistry & Engineering, 7(7), 6685–6695.
Kuo, C. H., Liu, Y. C., Chang, C. M. J., Chen, J. H., Chang, C., & Shieh, C. J. (2012). Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydrate Polymers, 87(4), 2538–2545.
Luo, Y., Jin, D., He, W., Huang, J., Chen, A., & Qi, F. (2021). A SiO2 microcarrier with an opal-like structure for cross-linked enzyme immobilization. Langmuir, 37(48), 14147–14156.
Cheng, Q. J., Chi, X. Y., Liang, Y. C., Li, W. X., Sun, J. X., Tao, J., & Wang, Z. (2023). immobilization of lipase in Cu-BTC MOF with enhanced catalytic performance for resolution of n-hydroxymethyl vince lactam. Applied Biochemistry and Biotechnology, 195, 1216–1230.
Coskun, G., Ciplak, Z., Yildiz, N., & Mehmetoglu, U. (2021). Immobilization of Candida antarctica lipase on nanomaterials and investigation of the enzyme activity and enantioselectivity. Applied Biochemistry and Biotechnology, 193(2), 430–445.
Rodrigues, R. C., Berenguer-Murcia, A., Carballares, D., Morellon-Sterling, R., & Fernandez-Lafuente, R. (2021). Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnology Advances, 52, 107821.
Tipton, K. F., & Dixon, H. (1979). Effects of pH on enzymes. Methods in Enzymology, 63, 183–234.
Xinyan, C., & B., He, M., Feng, D., Zhao, J., & Sun. (2020). Immobilized laccase on magnetic nanoparticles for enhanced lignin model compounds degradation. Chinese Journal of Chemical Engineering, 28(8), 165–172.
Guo, H., Lei, B., Yu, J., Chen, Y., & Qian, J. (2021). Immobilization of lipase by dialdehyde cellulose crosslinked magnetic nanoparticles. International journal of biological macromolecules, 185(11), 287–296.
Da, A., At, B., Dy, C., Sst, B., Fld, E. J. E., & Technology, M. (2020). Modified silicates and carbon nanotubes for immobilization of lipase from Rhizomucor miehei: Effect of support and immobilization technique on the catalytic performance of the immobilized biocatalysts. Enzyme and Microbial Technology, 144, 109739.
Iyer, P. V., & Ananthanarayan, L. (2008). Enzyme stability and stabilization-aqueous and non-aqueous environment. Process Biochemistry, 43(10), 1019–1032.
Funding
Financial support was provided by the Natural Science Foundation of China (NSFC) (No. 21978112) and MOE & SAFEA for the 111 Project (B13025).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. The main design of the article was performed by Pingbo Zhang. Material preparation, data collection, and analysis were performed by Mingming Fan and Pingping Jiang. The first draft of the manuscript was written by Xiulin Fan. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Consent to publish has been received from all participants.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, X., Zhang, P., Fan, M. et al. Effect of Glutaraldehyde Multipoint Covalent Treatments on Immobilized Lipase for Hydrolysis of Acidified Oil. Appl Biochem Biotechnol 195, 6942–6958 (2023). https://doi.org/10.1007/s12010-023-04477-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04477-y