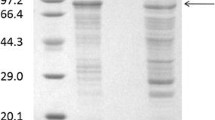
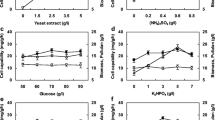
Abstract
Pullulanase is a debranching enzyme that cleaves explicitly α-1,6 glycosidic bonds, which is widely used in starch saccharification, production of glucose, maltose, and bioethanol. The thermal-resistant pullulanase is isolated from a variety of microorganisms; however, the lack of industrial production of pullulanase has hindered the transformation of the laboratory to industry. In this study, the expensive maltose syrup and soybean meal powder were replaced with cheap corn starch and corn steep liquor, exhibiting 440 U/mL of pullulanase in shake flasks by changing the C/N value and the total energy of the medium. Subsequently, the cultivation conditions were explored in a 50-L and 50-m3 bioreactor. In batch culture, the pullulanase activity reached 896 U/mL, while it increased to 1743 U/mL in fed-batch culture by controlling the dissolved oxygen, pH, reducing sugar content, and temperature. Remarkably, the cultivation volume was enlarged to 50 m3 based on the technical parameters of fed-batch culture. The industrial production of pullulanase was successful, and the activity achieved 1546 U/mL. When the product was stored at room temperature (25 °C) for 6 months, the pullulanase activity was over 90%. The half-lives at 60 and 80 °C were 119.45 h and 51.18 h, respectively, which satisfied the industrial application requirements of pullulanase.





Similar content being viewed by others
References
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D., & Pozzer, A. (2015). The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature, 525(7569), 367-+.
Sih, A., Ferrari, M. C. O., & Harris, D. J. (2011). Evolution and behavioural responses to human-induced rapid environmental change. Evolutionary Applications, 4(2), 367–387.
Agarwal, A. K. (2007). Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Progress in Energy and Combustion Science, 33(3), 233–271.
Alonso, D. M., Bond, J. Q., & Dumesic, J. A. (2010). Catalytic conversion of biomass to biofuels. Green Chemistry, 12(9), 1493–1513.
Alvira, P., Tomas-Pejo, E., Ballesteros, M., & Negro, M. J. (2010). Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresource Technology, 101(13), 4851–4861.
Mekonnen, M. M., Romanelli, T. L., Ray, C., Hoekstra, A. Y., Liska, A. J., & Neale, C. M. U. (2018). Water, energy, and carbon footprints of bioethanol from the US and Brazil. Environmental Science & Technology, 52(24), 14508–14518.
Lee, S. H., Shetty, J. K., & Teunissen, P. J. M. (2014). Single pH process for starch liquefaction and saccharification for high-density glucose syrups, US.
Li, C., Fang, D., Li, Z., Gu, Z., Yang, Q., Cheng, L., & Hong, Y. (2016). An improved two-step saccharification of high-concentration corn starch slurries by granular starch hydrolyzing enzyme. Industrial Crops and Products, 94, 259–265.
Borchert, M., Gjermansen, M., Clark, S., Henrissat, B., Silow, M.B., Hallin, P.F. (2014). Pullulanase variants and uses thereof, Novozymes AS.
Singh, R. S., Saini, G. K., & Kennedy, J. F. (2010). Maltotriose syrup preparation from pullulan using pullulanase. Carbohydrate Polymers, 80(2), 401–407.
Wang, X., Nie, Y., & Xu, Y. (2019). Industrially produced pullulanases with thermostability: discovery, engineering, and heterologous expression. Bioresource Technology, 278, 360–371.
Haki, G. D., & Rakshit, S. K. (2003). Developments in industrially important thermostable enzymes: a review. Bioresource Technology, 89(1), 17–34.
Xu, B. O., Yang, Y.-J., & Huang, Z.-X. (2006). Cloning and overexpression of gene encoding the pullulanase from Bacillus naganoensis in Pichia pastoris. Journal of Microbiology and Biotechnology, 16(8), 1184–1191.
Nie, Y., Yan, W., Xu, Chen, W. B., & Mu, X. (2013). High-level expression of Bacillus naganoensis pullulanase from recombinant Escherichia coli with auto-induction: effect of lac Operator. Plos One. 8(10), e78416.
Yao, N., Wei, Y., Yan, X., Bo, C. W., Mu, X. Q., Wang, X., Rong, X., & Riggs, P. D. (2013). High-level expression of Bacillus naganoensis pullulanase from recombinant Escherichia coli with auto-induction: effect of lac Operator. Plos One, 8(10), e78416.
Chen, A., Li, Y., Liu, X., Long, Q., Yang, Y., & Bai, Z. (2014). Soluble expression of pullulanase from Bacillus acidopullulyticus in Escherichia coli by tightly controlling basal expression. Journal of Industrial Microbiology & Biotechnology, 41(12), 1803–1810.
Andersen, C., Jorgensen, C.T., Bisgard-Frantzen, H., Svendsen, A., Kjaerulff, S. (2003). Alpha-amylase variants. Novozymes AS.
Shankar, R., Madihah, M. S., Shaza, E. M., Nur Aswati, K. O., Suraini, A. A., & Kamarulzaman, K. (2014). Application of different feeding strategies in fed batch culture for pullulanase production using sago starch. Carbohydrate Polymers, 102(Complete), 962–969.
Wang, Y., Chen, S., Zhao, X., Zhang, Y., & Yao, X. (2019). Enhancement of the production of Bacillus naganoensis pullulanase in recombinant Bacillus subtilis by integrative expression. Protein Expression & Purification. 159, 42–48.
Zhang, K., Su, L., Duan, X., Liu, L., & Wu, J. (2017). High-level extracellular protein production in Bacillus subtilis using an optimized dual-promoter expression system. Microbial Cell Factories, 16(1), 32.
Liu, X., Wang, H., Bin, W., & Pan, L. (2018). Efficient production of extracellular pullulanase in Bacillus subtilis ATCC6051 using the host strain construction and promoter optimization expression system. Microbial Cell Factories, 17(1), 163.
Meng, F., Zhu, X., Nie, T., Lu, F., Bie, X., Lu, Y., Trouth, F., & Lu, Z. (2018). Enhanced expression of pullulanase in Bacillus subtilis by new strong promoters mined from transcriptome data, both alone and in combination. Front Microbiol, 9, 2635.
Svendsen, A. (2008). Pullulanase variants and methods for preparing such variants with predetermined properties, Novozymes AS.
Svendsen, A. (2011). Pullulanase variants and methods for preparing such variants with predetermined properties, Novozymes AS.
Miller, B. S., & Shetty, J. K. (2008). Modified forms of pullulanase, US.
Fan, Y., Lu, Y., Du, X., Du, H., & Li, F. (2019). Truncated pullulanases, methods of production, and methods of use thereof, US.
Liaw, G. C., Pedersen, S., Hendriksen, H. V., Svendsen, A., Nielsen, B. R., & Nielsen, R. I. (2000). Method of producing saccharide preparations, Novo Nordisk A/S. Denmark: Bagsvaerd.
Sun, S., Zhang, L., Liu, F., Fan, X., & Sun, R. C. (2018). One-step process of hydrothermal and alkaline treatment of wheat straw for improving the enzymatic saccharification. Biotechnology for Biofuels, 11, 137.
Wang, X., Nie, Y., Mu, X., Xu, Y., & Xiao, R. (2016). Disorder prediction-based construct optimization improves activity and catalytic efficiency of Bacillus naganoensis pullulanase. Scientific Reports, 6(1), 24574.
Pang, B., Zhou, L., Cui, W., Liu, Z., Zhou, S., Xu, J., & Zhou, Z. (2019). A hyperthermostable type II pullulanase from a deep-sea microorganism Pyrococcus yayanosii CH1. Journal of Agricultural and Food Chemistry, 67(34), 9611–9617.
Bertoldo, C., Armbrecht, M., Becker, F., Schäfer, T., Antranikian, G., & Liebl, W. (2004). Cloning, sequencing, and characterization of a heat- and alkali-stable type i pullulanase from Anaerobranca gottschalkii. Appl Environ Microb, 70(6), 3407.
Duan, X., Chen, J., & Wu, J. (2013). Improving the thermostability and catalytic efficiency of Bacillus deramificans pullulanase by site-directed mutagenesis. Applied and Environmental Microbiology, 79(13), 4072–4077.
Yang, Y., Zhu, Y. Y., Obaroakpo, J. U., Zhang, S. W., Lu, J., Yang, L., Ni, D. W., Pang, X. Y., & Lv, J. P. (2020). Identification of a novel type I pullulanase from Fervidobacterium nodosum Rt17-B1, with high thermostability and suitable optimal pH. International Journal of Biological Macromolecules, 143, 424–433.
Funding
The authors acknowledge the financial support provided by the National Natural Science Foundation of China (31771948)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Statement
The research did not include any human subjects or animal experiments.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, F., Zhu, X., Zhao, H. et al. Improve Production of Pullulanase of Bacillus subtilis in Batch and Fed-Batch Cultures. Appl Biochem Biotechnol 193, 296–306 (2021). https://doi.org/10.1007/s12010-020-03419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03419-2