Abstract
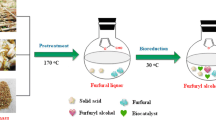
2,5-Furandicarboxylic acid (FDCA), one of the top biomass-based platform chemical, is highly promising for resins and polymers, and it can be prepared from the bio-oxidation of hydroxymethyl furfural (HMF), which can be obtained mainly from lignocellulosic glucose that has a high production potential from not edible biomass.
A native strain, Acinetobacter calcoaceticus NL14, that could convert HMF into FDCA is used for combining degradation and fermentation by consolidated bioprocessing (CBP). In this study, it was observed that the initial HMF concentration and pH neutralizer played important roles in the bioconversion of HMF, 5 g/L of HMF could be converted by 100% within 48 h with 0.5 g/L sodium carbonate (Na2CO3) with the production of 0.31 g/L FDCA. Extra glucose and hydrogen peroxide (H2O2) addition could further promote the production of FDCA to 0.54 g/L with 100% HMF conversion and a higher conversion rate. This report could provide a potential native bacterium for furan chemicals bioconversion and bioelimination, especially for FDCA bioproduction.





Similar content being viewed by others
References
Zhang, Y., Xia, C., Lu, M., & Tu, M. (2018). Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates. Biotechnology for Biofuels, 11(1), 178.
Quemeneur, M., et al. (2012). Inhibition of fermentative hydrogen production by lignocellulose-derived compounds in mixed cultures. International Journal of Hydrogen Energy, 37(4), 3150–3159.
Lewkowski, J. (2001). Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivatives. Arkivoc, 1, 17–54.
Rosatella, A. A., Simeonov, S. P., Frade, R. F. M., & Afonso, C. A. M. (2011). 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chemistry, 13(4), 754–793.
Werpy, T., & Petersen, G. (2004). Top value added chemicals from biomass: volume I--results of screening for potential candidates from sugars and synthesis gas. Golden: National Renewable Energy Lab.
Moreau, C., Belgacem, M. N., & Gandini, A. (2004). Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Topics in Catalysis, 27(1–4), 11–30.
Kamm, B. (2007). Production of platform chemicals and synthesis gas from biomass. Angewandte Chemie (International Ed. in English), 46(27), 5056–5058.
Teong, S. P., Yi, G. S., & Zhang, Y. G. (2014). Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chemistry, 16(4), 2015–2026.
Ardemani, L., Cibin, G., Dent, A. J., Isaacs, M. A., Kyriakou, G., Lee, A. F., Parlett, C. M. A., Parry, S. A., & Wilson, K. (2015). Solid base catalysed 5-HMF oxidation to 2,5-FDCA over Au/hydrotalcites: Fact or fiction? Chemical Science, 6(8), 4940–4945.
Carlini, C., Patrono, P., Galletti, A. M. R., Sbrana, G., & Zima, V. (2005). Selective oxidation of 5-hydroxymethyl-2-furaldehyde to furan-2,5-dicarboxaldehyde by catalytic systems based on vanadyl phosphate. Applied Catalysis A: General, 289(2), 197–204.
Gorbanev, Y. Y., et al. (2009). Gold-catalyzed aerobic oxidation of 5-hydroxymethylfurfural in water at ambient temperature.ChemSusChem: Chemistry & Sustainability Energy & Materials, 2(7), 672–675.
Thomas, S. M., DiCosimo, R., & Nagarajan, V. (2002). Biocatalysis: Applications and potentials for the chemical industry. Trends in Biotechnology, 20(6), 238–242.
Jimenez, D. J., Korenblum, E., & van Elsas, J. D. (2014). Novel multispecies microbial consortia involved in lignocellulose and 5-hydroxymethylfurfural bioconversion. Applied Microbiology and Biotechnology, 98(6), 2789–2803.
Wierckx, N., Koopman, F., Ruijssenaars, H. J., & de Winde, J. H. (2011). Microbial degradation of furanic compounds: Biochemistry, genetics, and impact. Applied Microbiology and Biotechnology, 92(6), 1095–1105.
Dijkman, W. P., & Fraaije, M. W. (2014). Discovery and characterization of a 5-hydroxymethylfurfural oxidase from Methylovorus sp. strain MP688. Applied and Environmental Microbiology, 80(3), 1082–1090.
Koopman, F., Wierckx, N., de Winde, J. H., & Ruijssenaars, H. J. (2010). Efficient whole-cell biotransformation of 5-(hydroxymethyl)furfural into FDCA, 2,5-furandicarboxylic acid. Bioresource Technology, 101(16), 6291–6296.
Ran, H., Zhang, J., Gao, Q., Lin, Z., & Bao, J. (2014). Analysis of biodegradation performance of furfural and 5-hydroxymethylfurfural by Amorphotheca resinae ZN1. Biotechnology for Biofuels, 7(1), 51.
Paller, G., Hommel, R. K., & Kleber, H. P. (1995). Phenol degradation by Acinetobacter calcoaceticus NCIB 8250. Journal of Basic Microbiology, 35(5), 325–335.
Lee, P., Hwang, Y., & Lee, T. (2019). Fermentative bio-hydrogen production of food waste in the presence of different concentrations of salt (Na(+)) and nitrogen. Journal of Microbiology and Biotechnology, 29(2), 283–291.
Almeida, J. R., et al. (2009). Metabolic effects of furaldehydes and impacts on biotechnological processes. Applied Microbiology and Biotechnology, 82(4), 625–638.
Tsuji, A., et al. (1982). The effects of temperature and pH on the growth of eight enteric and nine glucose non-fermenting species of gram-negative rods. Microbiology and Immunology, 26(1), 15–24.
Muller, R. H., & Babel, W. (1986). Glucose as an energy donor in acetate growing Acinetobacter-Calcoaceticus. Archives of Microbiology, 144(1), 62–66.
Muller, R. H., & Babel, W. (1984). Glucose as an auxiliary substrate - the influence of its carbon catabolism on the maximum carbon conversion efficiency. Applied Microbiology and Biotechnology, 20(3), 195–200.
Shoji, O., Fujishiro, T., Nakajima, H., Kim, M., Nagano, S., Shiro, Y., & Watanabe, Y. (2007). Hydrogen peroxide dependent monooxygenations by tricking the substrate recognition of cytochrome P450BSbeta. Angewandte Chemie (International Ed. in English), 46(20), 3656–3659.
Wang, B., Johnston, E. M., Li, P., Shaik, S., Davies, G. J., Walton, P. H., & Rovira, C. (2018). QM/MM studies into the H2O2-dependent activity of lytic polysaccharide monooxygenases: Evidence for the formation of a caged hydroxyl radical intermediate. ACS Catalysis, 8(2), 1346–1351.
Koopman, F., Wierckx, N., de Winde, J. H., & Ruijssenaars, H. J. (2010). Identification and characterization of the furfural and 5-(hydroxymethyl)furfural degradation pathways of Cupriavidus basilensis HMF14. Proceedings of the National Academy of Sciences of the United States of America, 107(11), 4919–4924.
Jiang, Y., Wilkins, P. C., & Dalton, H. (1993). Activation of the hydroxylase of sMMO from Methylococcus capsulatus (Bath) by hydrogen peroxide. Biochimica et Biophysica Acta, 1163(1), 105–112.
Funding
The research was supported by the financial support from the National Natural Science Foundation of China (31901270), the Scientific Research Start-up Funds of Nanjing Forestry University, China (163030127), the National Natural Science Funding of China (grant no. 31370573), and the Key Research and Development Program of Jiangsu (BE 2015758). This work is also financially supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheng, Y., Tan, X., Zhou, X. et al. Bioconversion of 5-Hydroxymethylfurfural (HMF) to 2,5-Furandicarboxylic Acid (FDCA) by a Native Obligate Aerobic Bacterium, Acinetobacter calcoaceticus NL14. Appl Biochem Biotechnol 192, 455–465 (2020). https://doi.org/10.1007/s12010-020-03325-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03325-7