Abstract
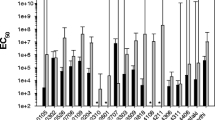
The polyphagous caterpillar, Spodoptera frugiperda, has been controlled with either chemical insecticides or transgenic plants such as Bt maize that expresses the cry and/or vip genes of the Bacillus thuringiensis (Bt) bacterium. Despite the efficiency of Bt toxins in lepidopteran control, populations resistant to Bt plants have emerged in different locations around the world. Thus, understanding how combined proteins interact against pests can assist resistance control and management. This work demonstrated the toxicity of Cry1Ab, Cry1Ac, Cry1Ca, Cry1Ea, Cry2Aa, Cry2Ab, Vip3Aa, and Vip3Ca in single and combined assays against S. frugiperda neonatal larvae. All protein mixtures had synergistic action in the control of the larvae. The Vip3Aa + Cry1Ab mixture had the highest toxicity, sequentially followed by Vip3Aa + Cry2Ab, Cry1Ab + Cry2Ab + Vip3Aa, Cry1Ea + Cry1Ca, Cry1Ab + Cry2Ab, Vip3Ca + Cry1Ea, and Vip3Ca + Cry1Ca. Cry1Ab, Cry1Ac, Cry2Ab, and Vip3Aa bound to more than one site on the brush border membrane vesicles (BBMV) of S. frugiperda. The Cry1Ab and Cry1Ac proteins share binding site, while Cry1Ab does not share binding site with the Cry2Aa and Cry2Ab proteins. The Vip3Aa protein does not share receptors with the tested Cry1 and Cry2. The results suggest that combination these tested proteins may increase toxicity against S. frugiperda neonates.


Similar content being viewed by others
References
Storer, N. P., Babcock, J. M., Schlenz, M., Meade, T., Thompson, G. D., Bing, J. W., & Huckaba, R. M. (2010). Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. Journal of Economic Entomology, 103(4), 1031–1038.
Storer, N. P., Kubiszak, M. E., King, J. E., Thompson, G. D., & Santos, A. C. (2012). Status of resistance to Bt maize in Spodoptera frugiperda: lessons from Puerto Rico. Journal of Invertebrate Pathology, 110(3), 294–300.
Farias, J. R., Andow, D. A., Horikoshi, R. J., Sorgatto, R. J., Fresia, P., dos Santos, A. C., & Omoto, C. (2014). Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Protection, 64, 150–158.
Omoto, C., Bernardi, O., Salmeron, E., Sorgatto, R. J., Dourado, P. M., Crivellari, A., Carvalho, R. B., Willse, A., Martinelli, S., & Head, G. P. (2016). Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Management Science, 72(9), 1727–1736.
Monnerat, R., Martins, E., Macedo, C., Queiroz, P., Praça, L., Soares, C. M., Moreira, H., Grisi, I., Silva, J., Soberon, M., & Bravo, A. (2015). Evidence of field-evolved resistance of Spodoptera frugiperda to Bt corn expressing Cry1F in Brazil that is still sensitive to modified Bt toxins. PLoS One, 10, 1–12.
Bernardi, O., Bernardi, D., Horikoshi, R. J., Okuma, D. M., Miraldo, L. L., Fatoretto, J., Medeiros, F. C., Burd, T., & Omoto, C. (2016). Selection and characterization of resistance to the Vip3Aa20 protein from Bacillus thuringiensis in Spodoptera frugiperda. Pest Management Science, 72(9), 1794–1802.
Horikoshi, R. J., Bernardi, D., Bernardi, O., Malaquias, J. B., Okuma, D. M., Miraldo, L. L., Amaral, F. A., & Omoto, C. (2016). Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: implications for resistance management. Scientific Reports, 6(1). https://doi.org/10.1038/srep34864.
Lee, M. K., Walters, F. S., Hart, H., Palekar, N., & Chen, J. S. (2003). The mode of action of the Bacillus thuringiensis vegetative insecticidal protein Vip3A differs from that of Cry1Ab δ-endotoxin. Applied and Environmental Microbiology, 69(8), 4648–4657.
de Maagd, R. A., Bravo, A., Berry, C., Crickmore, N., & Schnepf, H. E. (2003). Structure, diversity and evolution of protein toxins from sporeforming entomopathogenic bacteria. Annual Review of Genetics, 37(1), 409–433.
Pardo-López, L., Soberón, M., & Bravo, A. (2013). Bacillus thuringiensis insecticidal 3-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiology Reviews, 37(1), 3–22.
Sena, J. A., Hernández-Rodríguez, C. S., & Ferré, J. (2009). Interaction of Bacillus thuringiensis Cry1 and Vip3A proteins with Spodoptera frugiperda midgut binding sites. Applied and Environmental Microbiology, 75(7), 2236–2237.
Tabashnik, B. E. (2015). ABCs of insect resistance to Bt. PLoS Genetics, 11(11), e1005646.
Ocelotl, J., Sánchez, J., Arroyo, R., García-Gómez, B. I., Gómez, I., Unnithan, G. C., Tabashnik, B. E., Bravo, A., & Soberón, M. (2015). Binding and oligomerization of modified and native Bt toxins in resistant and susceptible pink bollworm. PLoS One, 10(12), e0144086.
Abdelkefi-Mesrati, L., Boukedi, H., Dammak-Karray, M., Sellami-Boudawara, T., Jaoua, S., & Tounsi, S. (2011). Study of the Bacillus thuringiensis Vip3Aa16 histopathological effects and determination of its putative binding proteins in the midgut of Spodoptera littoralis. Journal of Invertebrate Pathology, 106(2), 250–254.
Chakroun, M., & Ferré, J. (2014). In vivo and in vitro binding of Vip3Aa to Spodoptera frugiperda midgut and characterization of binding sites by 125I radiolabeling. Applied and Environmental Microbiology, 80(20), 6258–6265.
Sellami, S., Cherif, M., Abdelkefi-Mesrati, L., Tounsi, S., & Jamoussi, K. (2015). Toxicity, activation process, and histopathological effect of Bacillus thuringiensis vegetative insecticidal protein Vip3Aa16 on Tuta absoluta. Applied Biochemistry and Biotechnology, 175(4), 1992–1999.
Jiang, K., Mei, S. Q., Wang, T. T., Pan, J. H., Chen, Y. H., & Cai, J. (2016). Vip3Aa induces apoptosis in cultured Spodoptera frugiperda (Sf9) cells. Toxicon, 120, 49–56.
Ferré, J., & Van Rie, J. (2002). Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annual Review of Entomology, 47(1), 501–533.
Heckel, D. G. (2015). Roles of ABC proteins in the mechanism and management of Bt resistance. In M. Soberón, Y. Gao, & A. Bravo (Eds.), Bt resistance—characterization and strategies for GM crops producing Bacillus thuringiensis toxins (pp. 138–149). Boston: CABI Biotechnology.
Carriere, Y., Fabrick, J. A., & Tabashnik, B. E. (2016). Can pyramids and seed mixtures delay resistance to Bt crops? Trends in Biotechnology, 34(4), 291–302.
Lemes, A. R. N., Davolos, C. C., Legori, P. C. B. C., Fernandes, O. A., Ferre, J., Lemos, M. V. F., & Desiderio, J. A. (2014). Synergism and antagonism between Bacillus thuringiensis Vip3A and Cry1 proteins in Heliothis virescens, Diatraea saccharalis and Spodoptera frugiperda. PloS One, 9(10), e107196.
Ricietto, A. P. S., Gomis-Cebolla, J., Vilas-Bôas, G. T., & Ferré, J. (2016). Susceptibility of Grapholita molesta (Busck, 1916) to formulations of Bacillus thuringiensis, individual toxins and their mixtures. Journal of Invertebrate Pathology, 141, 1–5.
Bergamasco, V. B., Mendes, D. R. P., Fernandes, O. A., Desidério, J. A., & Lemos, M. V. F. (2013). Bacillus thuringiensis Cry1Ia10 and Vip3Aa protein interactions and their toxicity in Spodoptera spp.(Lepidoptera). Journal of Invertebrate Pathology, 112(2), 152–158.
Herrero, S., González-Cabrera, J., Ferre, J., Bakker, P. L., & De Maagd, R. A. (2004). Mutations in the Bacillus thuringiensis Cry1Ca toxin demonstrate the role of domains II and III in specificity towards Spodoptera exigua larvae. The Biochemical Journal, 384(3), 507–513.
Figueiredo, C. S., Marucci, S. C., Tezza, R. I. D., Lemos, M. V. F., & Desidério, J. A. (2013). Caracterização do gene vip3A e toxicidade da proteína Vip3Aa50 á lagarta-do-cartucho e á lagarta-da-soja. Pesquisa Agropecuária Brasileira, 48(9), 1220–1227.
Palma, L., Hernández-Rodríguez, C. S., Maeztu, M., Hernández-Martínez, P., Escudero, I. R., Escriche, B., Muñoz, D., Van Rie, J., Ferré, J., & Caballero, P. (2012). Vip3C, a novel class of vegetative insecticidal proteins from Bacillus thuringiensis. Applied and Environmental Microbiology, 78(19), 7163–7165.
Monnerat, R. Q., Batista, A. C., Medeiros, P. T., Martins, E. S., Melatti, V. M., Praça, L. B., Dumas, V. F., Morinaga, C., Demo, C., Gomes, A. C. M., Falcão, R., Siqueira, C. B., Silva-Werneck, J. O., & Berry, C. (2007). Screening of Brazilian Bacillus thuringiensis isolates active against Spodoptera frugiperda, Plutella xylostella and Anticarsia gemmatalis. Biological Control, 41(3), 291–295.
Tabashnik, B. E. (1992). Evaluation of synergism among Bacillus thuringiensis toxins. Applied and Environmental Microbiology, 58, 3343–3346.
Finney, D. J. (1971). Probit analysis. London: Cambridge University Press.
Wolfersberger, M. G., Luethy, P., Maurer, A., Parenti, P., Sacchi, V. F., Giordana, B., & Hanozet, G. M. (1987). Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pierisbrassicae). Comparative Biochemistry and Physiology, 86(2), 301–308.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Chakroun, M., & Ferré, J. (2014). In vivo and in vitro binding of Vip3Aa to Spodoptera frugiperda midgut and characterization of binding sites using 125I-radiolabeling. Applied and Environmental Microbiology, 80(20), 6258–6265.
Bernardi, O., Amado, D., Sousa, R. S., Segatti, F., Fatoretto, J., Burd, A. D., & Omoto, C. (2014). Baseline susceptibility and monitoring of Brazilian populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea saccharalis (Lepidoptera: Crambidae) to Vip3Aa20 insecticidal protein. Journal of Economic Entomology, 107(2), 781–790.
Lemes, N. A., Figueiredo, C. S., Sebastião, I., Silva, L. M., Alves, R. C., Siqueira, H. A. A., Lemos, M. V. F., Fernandes, O. A., & Desidério, J. A. (2017). Cry1Ac and Vip3Aa proteins from Bacillus thuringiensis targeting Cry toxin resistance in Diatraea flavipennella and Elasmopalpus lignosellus from sugarcane. PeerJ, 5, e2866.
Yang, J., Quan, Y., Sivaprasath, P., Shabbir, M. Z., Wang, Z., Ferré, J., & He, K. (2018). Insecticidal activity and synergistic combinations of ten different Bt toxins against Mythimna separata (Walker). Toxins (Basel)., 10(11).
Lee, M. K., Curtiss, A., Alcantara, E., & Dean, D. H. (1996). Synergistic effect of the Bacillus thuringiensis toxins CryIAa and CryIAc on the gypsy moth, Lymantria dispar. Applied and Environmental Microbiology, 62, 583–586.
Muñóz-Garay, C., Portugal, L., Pardo-López, L., Jiménez-Juárez, N., Arenas, I., Gómez, I., Sánchez-López, R., Arroyo, R., Holzenburg, A., Savva, C. G., Soberón, M., & Bravo, A. (2009). Characterization of the mechanism of action of the genetically modifi ed Cry1AbMod toxin that is active against Cry1Ab-resistant insects. Biochimica et Biophysica Acta, 1788(10), 2229–2237.
Carmona, D., Rodríguez-Almazán, C., Muñoz-Garay, C., Portugal, L., Pérez, C., De Maagd, R. A., et al. (2011). Dominant negative phenotype of Bacillus thuringiensis Cry1Ab, Cry11Aa and Cry4Ba mutants suggest hetero-oligomer formation among different cry toxins. PLoS One, 6(5), e19952.
Kunthic, T., Surya, W., & Promdonkoy, B. (2017). Conditions for homogeneous preparation of stable monomeric and oligomeric forms of activated Vip3A toxin from Bacillus thuringiensis. European Biophysics Journal, 46(3), 257–264.
Herrero, S., Bel, Y., Hernández-Martínez, P., & Ferré, J. (2016). Susceptibility, mechanisms of response and resistance to Bacillus thuringiensis toxins in Spodoptera spp. Current Opinion in Insect Science, 15, 89–96.
Osman, G. H., Altaf, W. J., Saleh, I. A. S., Soltane, R., Abulreesh, H. H., Arif, I. A., Ramadan, A. M., & Osman, Y. A. (2018). First report of detection of the putative receptor of Bacillus thuringiensis toxin Vip3Aa from black cutworm (Agrotis ipsilon). Saudi Journal of Biological Sciences, 25(3), 441–445.
Qiu, L., Zhang, B., Liu, L., Ma, W., Wang, X., Lei, C., & Chen, L. (2017). Proteomic analysis of Cry2Aa-binding proteins and their receptor function in Spodoptera exigua. Scientific Reports, 7(1), 40222.
Jurat-Fuentes, J. L., & Adang, M. J. G. (2001). Importance of Cry1 d-endotoxin domain II loops for binding specificity in Heliothis virescens (L.). Applied and Environmental Microbiology, 67(1), 323–329.
Rajagopal, R., Agrawal, N., Selvapandiyan, A., Sivakumar, S., Ahmad, S., & Bhatnagar, R. K. (2003). Recombinantly expressed isoenzymic aminopeptidases from Helicoverpa armigera (American cotton bollworm) midgut display differential interaction with closely related Bacillus thuringiensis insecticidal proteins. The Biochemical Journal, 370(3), 971–978.
Hua, G., Jurat-Fuentes, J. L., & Adang, M. J. (2004). Fluorescent-based assays establish Manduca sexta Bt-R1a cadherin as a receptor for multiple Bacillus thuringiensis Cry1A toxins in Drosophila S2 cells. Insect Biochemistry and Molecular Biology, 34(3), 193–202.
Jurat-Fuentes, J. L., & Adang, M. J. (2006). The Heliothis virescens cadherin protein expressed in Drosophila S2 cells functions as a receptor for Bacillus thuringiensis Cry1A but not Cry1Fa toxins. Biochemistry, 45(32), 9688–9695.
Pacheco, S., Gómez, I., Gill, S. S., Bravo, A., & Soberón, M. (2009). Enhancement of insecticidal activity of Bacillus thuringiensis Cry1A toxins by fragments of a toxin-binding cadherin correlates with oligomer formation. Peptides, 30(3), 583–588.
Baxter, S. W., Badenes-Peréz, F. R., Morrison, A., Vogel, H., Crickmore, N., Kain, W., Wang, P., Heckel, D. G., & Jiggins, C. D. (2011). Parallel evolution of Bacillus thuringiensis toxin resistance in Lepidoptera. Genetics, 189(2), 675–679.
Da Silva, I. H. S., Goméz, I., Sánchez, J., Martínez de Castro, D. L., Valicente, F. H., Soberón, M., et al. (2018). Identification of midgut membrane proteins from different instars of Helicoverpa armigera (Lepidoptera: Noctuidae) that bind to Cry1Ac toxin. PLoS One, 13(12), e0207789.
Qiu, L., Hou, L., Zhang, B., Liu, L., Li, B., Deng, P., Ma, W., Wang, X., Fabrick, J. A., & Chen, L. (2015). Cadherin is involved in the action of Bacillus thuringiensis toxins Cry1Ac and Cry2Aa in the beet armyworm, Spodoptera exigua. Journal of Invertebrate Pathology, 127, 47–53.
Hernández-Rodríguez, C. S., Hernández-Martínez, P., van Rie, J., Escriche, B., & Ferré, J. (2013). Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS One, 8(7), e68164.
Lee, M. K., Miles, P., & Chen, J. (2006). Brush border membrane binding properties of Bacillus thuringiensis Vip3A toxin to Heliothis virescens and Helicoverpa zea midguts. Biochemical and Biophysical Research Communications, 339(4), 1043–1047.
Acknowledgments
Thanks are due to Dr. Ruud A. de Maagd (Plant Research International—Wageningen, Netherlands) for providing the clones carrying the cry1 genes and to Dr. Juan Ferré, University of Valencia, Campus de Burjassot, Spain, for providing the clone carrying the vip3Ca gene.
Funding
This work was financially supported by the São Paulo State Foundation for the Research Support (FAPESP) (grant support process no. 2013/15128-2) and the CAPES–Brazilian Federal Agency for Support and Evaluation of Graduate Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soares Figueiredo, C., Nunes Lemes, A.R., Sebastião, I. et al. Synergism of the Bacillus thuringiensis Cry1, Cry2, and Vip3 Proteins in Spodoptera frugiperda Control. Appl Biochem Biotechnol 188, 798–809 (2019). https://doi.org/10.1007/s12010-019-02952-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02952-z