Abstract
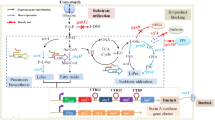
The membrane-bound gluconate dehydrogenase (mGADH) is a critical enzyme for 2-keto-d-gluconic acid (2KGA) production in Pseudomonas plecoglossicida JUIM01. The purified native flavin adenine dinucleotide-dependent mGADH (FAD-mGADH) was consisted of a gamma subunit, a flavoprotein subunit, and a cytochrome c subunit with molecular mass of ~ 27, 65, and 47 kDa, respectively. The specific activity of FAD-mGADH was determined as 90.71 U/mg at optimum pH and temperature of 6.0 and 35 °C. The Km and Vmax values of calcium d-gluconate were 0.631 mM and 0.734 mM/min. The metal ions Mg2+ and Mn2+ showed slight positive effects on FAD-mGADH activity. On the other hand, a 3868-bp-length gad gene cluster was amplified and expressed in Escherichia coli BL21(DE3). The recombinant protein showed the same molecular weight and enzyme activity as the native FAD-mGADH, which confirmed it as a FAD-mGADH encoding gene. The flavoprotein subunit and the cytochrome c subunit containing a putative FAD-binding motif and three possible heme-binding motifs concluded from alignment results of mGADHs. This study characterized the native and recombinant FAD-mGADH and would provide the basis for further genetic modification of Pseudomonas plecoglossicida JUIM01 with the intention of 2KGA productivity improvement.







Similar content being viewed by others
References
Stottmeister, U., Aurich, A., Wilde, H., Andersch, J., Schmidt, S., & Sicker, D. (2005). White biotechnology for green chemistry: fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses. Journal of Industrial Microbiology & Biotechnology, 32(11-12), 651–664. https://doi.org/10.1007/s10295-005-0254-x.
Pappenberger, G., & Hohmann, H. P. (2014). Industrial production of l-ascorbic acid (vitamin C) and d-isoascorbic acid. Advances in Biochemical Engineering/Biotechnology, 143, 143–188. https://doi.org/10.1007/10_2013_243.
Sun, W. J., Liu, C. F., Yu, L., Cui, F. J., Zhou, Q., Yu, S. L., & Sun, L. (2012). A novel bacteriophage KSL-1 of 2-keto-gluconic acid producer Pseudomonas fluorescens K1005: isolation, characterization and its remedial action. BMC Microbiology, 12(1), 127. https://doi.org/10.1186/1471-2180-12-127.
Sun, W. J., Zhou, Y. Z., Zhou, Q., Cui, F. J., Yu, S. L., & Sun, L. (2012). Semi-continuous production of 2-keto-gluconic acid by Pseudomonas fluorescens AR4 from rice starch hydrolysate. Bioresource Technology, 110, 546–551. https://doi.org/10.1016/j.biortech.2012.01.040.
Umezawa, K., Takeda, K., Ishida, T., Sunagawa, N., Makabe, A., Isobe, K., Koba, K., Ohno, H., Samejima, M., Nakamura, N., Igarashi, K., & Yoshida, M. (2015). A novel pyrroloquinoline quinone-dependent 2-keto-d-glucose dehydrogenase from Pseudomonas aureofaciens. Journal of Bacteriology, 197(8), 1322–1329. https://doi.org/10.1128/JB.02376-14.
Chia, M., Van Nguyen, T. B., & Choi, W. J. (2008). DO-stat fed-batch production of 2-keto-d-gluconic acid from cassava using immobilized Pseudomonas aeruginosa. Applied Microbiology and Biotechnology, 78(5), 759–765. https://doi.org/10.1007/s00253-008-1374-9.
Sun, W. J., Xiao, F. F., Wei, Z., Cui, F. J., Yu, L., Yu, S. L., & Zhou, Q. (2015). Non-sterile and buffer-free bioconversion of glucose to 2-keto-gluconic acid by using Pseudomonas fluorescens AR4 free resting cells. Process Biochemistry, 50(4), 493–499. https://doi.org/10.1016/j.procbio.2015.01.011.
Wei, Z., Yu, S. L., Sun, W. J., Zhou, Q., & Li, Z. B. (2008). Research progress on fermentation production of 2-keto-d-gluconic acid. Food Science, 29, 636–639 (in Chinese).
Xue, Q., Wei, Z., Sun, W. J., Cui, F. J., Yu, S. L., Zhou, Q., & Liu, J. Z. (2015). 2-Keto-d-gluconate-yielding membrane-bound d-glucose dehydrogenase from Arthrobacter globiformis C224: purification and characterization. Molecules, 20(1), 846–862. https://doi.org/10.3390/molecules20010846.
Yang, G. F., Wei, Z., Sun, W. J., Cui, F. J., Wang, D. M., Yu, S. L., & Zhou, Q. (2015). Purification and enzymatic characterization of membrane-bound d-gluconate dehydrogenase from Arthrobacter globiformis. Journal of Molecular Catalysis B: Enzymatic, 113, 14–22. https://doi.org/10.1016/j.molcatb.2014.12.014.
Sun, W. J., Yun, Q. Q., Zhou, Y. Z., Cui, F. J., Yu, S. L., Zhou, Q., & Sun, L. (2013). Continuous 2-keto-gluconic acid (2KGA) production from corn starch hydrolysate by Pseudomonas fluorescens AR4. Biochemical Engineering Journal, 77, 97–102. https://doi.org/10.1016/j.bej.2013.05.010.
Ramakrishnan, T., & Campbell, J. J. (1955). Gluconic dehydrogenase of Pseudomonas aeruginosa. Biochimica et Biophysica Acta, 17, 122–127. https://doi.org/10.1016/0006-3002(55)90326-7.
Matsushita, K., Shinagawa, E., Adachi, O., & Ameyama, M. (1979). Membrane-bound d-gluconate dehydrogenase from Pseudomonas aeruginosa: purification and structure of cytochrome-binding form. Journal of Biochemistry, 85, 1173–1181. https://doi.org/10.1093/oxfordjournals.jbchem.a132441.
Matsushita, K., Shinagawa, E., & Ameyama, M. (1982). D-Gluconate dehydrogenase from bacteria, 2-keto-d-gluconate-yielding, membrane-bound. Methods in Enzymology, 89, 187–193. https://doi.org/10.1016/S0076-6879(82)89033-2.
Toyama, H., Furuya, N., Saichana, I., Ano, Y., Adachi, O., & Matsushita, K. (2007). Membrane-bound, 2-keto-d-gluconate-yielding d-gluconate dehydrogenase from “Gluconobacter dioxyacetonicus” IFO 3271: molecular properties and gene disruption. Applied and Environmental Microbiology, 73(20), 6551–6556. https://doi.org/10.1128/AEM.00493-07.
Shinagawa, E., Matsushita, K., Adachi, O., & Ameyama, M. (1984). d-Gluconate dehydrogenase, 2-keto-d-gluconate yielding, from Gluconobacter dioxyacetonicus: purification and characterization. Agricultural and Biological Chemistry, 48(6), 1517–1522. https://doi.org/10.1271/bbb1961.48.1517.
Shinagawa, E., Matsushita, K., Adachi, O., & Ameyama, M. (1978). Membrane-bound d-gluconate dehydrogenase of Serratia marcescens: purification and properties. Agricultural and Biological Chemistry, 42(12), 2355–2361. https://doi.org/10.1080/00021369.1978.10863360.
Yum, D. Y., Lee, Y. P., & Pan, J. G. (1997). Cloning and expression of a gene cluster encoding three subunits of membrane-bound gluconate dehydrogenase from Erwinia cypripedii ATCC 29267 in Escherichia coli. Journal of Bacteriology, 179(21), 6566–6572. https://doi.org/10.1128/jb.179.21.6566-6572.1997.
Saichana, I., Moonmangmee, D., Adachi, O., Matsushita, K., & Toyama, H. (2009). Screening of thermotolerant Gluconobacter strains for production of 5-keto-d-gluconic acid and disruption of flavin adenine dinucleotide-containing d-gluconate dehydrogenase. Applied and Environmental Microbiology, 75(13), 4240–4247. https://doi.org/10.1128/AEM.00640-09.
Sun, W. J., Luan, F., Wang, D. M., Zhang, X. F., Cui, F. J., & Li, Y. Z. (2016). Cloning and bioinformatic analysis of a 2-ketogluconate transporter gene from Pseudomonas plecoglossicida. Modern Food Science and Technology, 50-55(in Chinese), 32. https://doi.org/10.13982/j.mfst.1673-9078.2016.6.009.
Wang, D. M., Sun, L., Sun, W. J., Cui, F. J., Gong, J. S., Zhang, X. M., Shi, J. S., & Xu, Z. H. (2018). Purification, characterization and gene identification of a membrane-bound glucose dehydrogenase from 2-keto-d-gluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01. International Journal of Biological Macromolecules, 118, 534–541. https://doi.org/10.1016/j.ijbiomac.2018.06.097.
Kobayashi, K., Mustafa, G., Tagawa, S., & Yamada, M. (2005). Transient formation of a neutral ubisemiquinone radical and subsequent intramolecular electron transfer to pyrroloquinoline quinone in the Escherichia coli membrane-integrated glucose dehydrogenase. Biochemistry, 44(41), 13567–13572. https://doi.org/10.1021/bi051347n.
Sara-Paez, M., Contreras-Zentella, M., Gomez-Manzo, S., Gonzalez-Valdez, A. A., Gasca-Licea, R., Mendoza-Hernandez, G., Escamilla, J. E., & Reyes-Vivas, H. (2015). Purification and characterization of the membrane-bound quinoprotein glucose dehydrogenase of Gluconacetobacter diazotrophicus PAL 5. The Protein Journal, 34(1), 48–59. https://doi.org/10.1007/s10930-014-9596-4.
Marchler-Bauer, A., Bo, Y., Han, L., He, J., Lanczycki, C. J., Lu, S., Chitsaz, F., Derbyshire, M. K., Geer, R. C., Gonzales, N. R., Gwadz, M., Hurwitz, D. I., Lu, F., Marchler, G. H., Song, J. S., Thanki, N., Wang, Z., Yamashita, R. A., Zhang, D., Zheng, C., Geer, L. Y., & Bryant, S. H. (2017). CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Research, 45(D1), D200–D203. https://doi.org/10.1093/nar/gkw1129.
Bendtsen, J. D., Nielsen, H., von Heijne, G., & Brunak, S. (2004). Improved prediction of signal peptides: SignalP 3.0. Journal of Molecular Biology, 340(4), 783–795. https://doi.org/10.1016/j.jmb.2004.05.028.
Chang, T. H., Huang, H. Y., Hsu, J. B., Weng, S. L., Horng, J. T., & Huang, H. D. (2013). An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs. BMC Bioinformatics, 14(Suppl 2), S4–S8. https://doi.org/10.1186/1471-2105-14-S2-S4.
Solovyev, V. S. A., & Salamov, A. (2011). Automatic annotation of microbial genomes and metagenomic sequences. in metagenomics and its applications in agriculture, biomedicine and environmental studies (Li, R.W., ed.), Nova Science Publishers, Hauppauge, NY, pp. 61–78.
Landete, J. M., Rodriguez, H., Curiel, J. A., de las Rivas, B., Mancheno, J. M., & Munoz, R. (2010). Gene cloning, expression, and characterization of phenolic acid decarboxylase from Lactobacillus brevis RM84. Journal of Industrial Microbiology & Biotechnology, 37(6), 617–624. https://doi.org/10.1007/s10295-010-0709-6.
Schmid, R. D., & Urlacher, V. B. (2007). Modern biooxidation: enzymes, reactions and applications. Hoboken, NJ: John Wiley & Sons.
Shinagawaa, E., Matsushitab, K., Toyamab, H., & Adachib, O. (1999). Production of 5-keto-d-gluconate by acetic acid bacteria is catalyzed by pyrroloquinoline quinone (PQQ)-dependent membrane-bound d-gluconate dehydrogenase. Journal of Molecular Catalysis B: Enzymatic, 6(3), 341–350. https://doi.org/10.1016/S1381-1177(98)00112-X.
Klasen, R., Bringer-Meyer, S., & Sahm, H. (1995). Biochemical characterization and sequence analysis of the gluconate: NADP 5-oxidoreductase gene from Gluconobacter oxydans. Journal of Bacteriology, 177(10), 2637–2643. https://doi.org/10.1128/jb.177.10.2637-2643.1995.
Prakash, B., Vidyasagar, M., Jayalakshmi, S. K., & Sreeramulu, K. (2012). Purification and some properties of low-molecular-weight extreme halophilic xylanase from Chromohalobacter sp. TPSV 101. Journal of Molecular Catalysis B: Enzymatic, 74(3-4), 192–198. https://doi.org/10.1016/j.molcatb.2011.10.004.
Zhang, W., Yan, B., Wang, J., Yao, J., & Yu, Z. (2006). Purification and characterization of membrane-bound l-sorbose dehydrogenase from Gluconobacter oxydans GO112. Enzyme and Microbial Technology, 38(5), 643–648. https://doi.org/10.1016/j.enzmictec.2005.07.016.
Acknowledgements
Many thanks go to Ph.D. Xue Cai (Jiangnan University) for her help regarding the language polish of this manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (31571885), the Innovation Group Construction Program of Jiangxi Province (20142BCB24024), the Science & Technology Program of Jiangxi Province (No. [2015]64), Science & Technology Program of Dexing city (No. [2015]44), and the National First-class Discipline Program of Light Industry Technology and Engineering (LITE2018-11 and LITE2018-18).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 4163 kb)
Rights and permissions
About this article
Cite this article
Wang, DM., Sun, L., Sun, WJ. et al. A Membrane-Bound Gluconate Dehydrogenase from 2-Keto-d-Gluconic Acid Industrial Producing Strain Pseudomonas plecoglossicida JUIM01: Purification, Characterization, and Gene Identification. Appl Biochem Biotechnol 188, 897–913 (2019). https://doi.org/10.1007/s12010-019-02951-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02951-0