Abstract
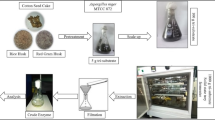
Due to great interest on producing bioactive compounds for functional foods and biopharmaceuticals, it is important to explore the microbial degradation of potential sources of target biomolecules. Gallotannins are polyphenols present in nature, an example of them is tannic acid which is susceptible to enzymatic hydrolysis. This hydrolysis is performed by tannase or tannin acyl hydrolase, releasing in this way, biomolecules with high-added value. In the present study, chemical profiles obtained after fungal degradation of tannic acid under two bioprocesses (submerged fermentation (SmF) and solid state fermentation (SSF)) were determined. In both fermentation systems (SmF and SSF), Aspergillus niger GH1 strain and tannic acid as a sole carbon source and inducer were used (the presence of tannic acid promotes production of enzyme tannase). In case of SSF, polyurethane foam (PUF) was used like as support of fermentation; culture medium only was used in case of submerged fermentation. Fermentation processes were monitored during 72 h; samples were taken kinetically every 8 h; and all extracts obtained were partially purified to obtain polyphenolic fraction and then were analyzed by liquid chromatography-mass spectrometry (LC-MS). Molecules like gallic acid and n-galloyl glucose were identified as intermediates in degradation of tannic acid; during SSF was identified ellagic acid production. The results obtained in this study will contribute to biotechnological production of ellagic acid.




Similar content being viewed by others
References
Bhat, T. K., Singh, B., & Sharma, O. P. (1998). Microbial degradation of tannins—a current perspective. Biodegradation, 9(5), 343–357. https://doi.org/10.1023/A:1008397506963.
Goldstein, J. L., & Swain, T. (1965). The inhibition of enzymes by tannins. Phytochemistry, 4(1), 185–192. https://doi.org/10.1016/S0031-9422(00)86162-2.
Haslam, E. (1989). Plant polyphenols—vegetable tannins revised. Cambridge: Cambridge University Press.
Mueller-Harvey, I. (1984). Analysis of hydrolysable tannins. Animal Feed Science and Technology, 91, 3–20.
Schofield, P., Mbugua, D. M., & Pell, A. N. (2001). Analysis of condensed tannins a review. Animal Feed Science and Technology, 91(1-2), 21–40. https://doi.org/10.1016/S0377-8401(01)00228-0.
Rodríguez, H., de las Rivas, B., Gómez-Córdovés, C., & Muñoz, R. (2008). Degradation of tannic acid by cell free extracts of Lactobacillus plantarum. Food Chemistry, 107(2), 664–670. https://doi.org/10.1016/j.foodchem.2007.08.063.
Ajay Kumar, R., Gunasekaran, P., & Lakshmanan, M. (1999). Biodegradation of tannic acid by Citrobacter freundii isolated from a tannery effluent. Journal of Basic Microbiology, 39(3), 161–168. https://doi.org/10.1002/(SICI)1521-4028(199906)39:3<161::AID-JOBM161>3.0.CO;2-U.
Aguilar, C. N., Rodríguez, R., Gutiérrez-Sánchez, G., Augur, C., Favela-Torres, E., Prado-Barragán, L. A., Ramírez-Coronel, A., & Contreras-Esquivel, J. C. (2007). Microbial tannases: advances and perspectives. Applied Microbiology and Biotechnology, 76(1), 47–59.
Haslam, E., & Stangroom, J. E. (1966). The esterase and depsidase activities of tannase. The Biochemical Journal, 99(1), 28–31. https://doi.org/10.1042/bj0990028.
Barbehenn, R. V., Jones, C. P., Hagerman, A. E., Karonen, M., & Salminen, J. P. (2004). Ellagitannins have larger oxidative activities than condensed tannins and galloyl glucoses at high pH: potential impact on caterpillars. Journal of Chemical Ecology, 2253–2267.
Hathway, D. E. (1957). The transformation of gallates into ellagate. The Biochemical Journal, 67(3), 445–450. https://doi.org/10.1042/bj0670445.
Mayer, W., Hoffmann, E. H., Lösch, N., Wolf, H., Wolter, B., & Schilling, G. (2001). Dehydrierungsreaktionen mit Gallussäureestern. Liebigs Annalen der Chemie, 1984, 929–938.
Chávez-González, M. L., Guyot, S., Rodríguez-Herrera, R., Prado-Barragán, A., & Aguilar, C. N. (2014). Production profiles of phenolics from fungal tannic acid biodegradation in submerged and solid-state fermentation. Process Biochemistry, 49(4), 541–546. https://doi.org/10.1016/j.procbio.2014.01.031.
Niemetz, R., & Gross, G. G. (2003). Oxidation of pentagalloylglucose to the ellagitannn, tellimagrandin II, by a phenol oxidase from Tellina grandiflora leaves. Phytochemistry, 62(3), 301–306. https://doi.org/10.1016/S0031-9422(02)00557-5.
Balakrishnan, K., & Pandey, A. (1996). Production of biologically active secondary metabolites in solid state fermentation. Journal of Scientific and Industrial Research, 55, 365–372.
Bigelis, R., He, H., Yang, H., Chang, L. P., & Greenstein, M. (2006). Production of fungal antibiotics using polymeric solid supports in solid-state and liquid fermentation. Journal of Industrial Microbiology & Biotechnology, 33(10), 815–826. https://doi.org/10.1007/s10295-006-0126-z.
Barrios-González, J. (2012). Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochemistry, 47(2), 175–185. https://doi.org/10.1016/j.procbio.2011.11.016.
Barrios-González, J., & Mejía, A. (2007). Production of antibiotics and other commercially valuable secondary metabolites. In A. Pandey, C. Larroche, C. R. Soccol, & J. A. Rodríguez-León (Eds.), Current developments in solid-state fermentation (pp. 262–296). New York/New Delhi: Springer Science/Asiatech Publishers, Inc.
Acknowledgements
The authors would like to gratefully acknowledge Mexican Council of Science and Technology (CONACYT) for the financial support for this study. MLCG thanks CONACYT for the financial support during her postgraduate studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Chávez-González, M.L., Guyot, S., Rodríguez-Herrera, R. et al. Exploring the Degradation of Gallotannins Catalyzed by Tannase Produced by Aspergillus niger GH1 for Ellagic Acid Production in Submerged and Solid-State Fermentation. Appl Biochem Biotechnol 185, 476–483 (2018). https://doi.org/10.1007/s12010-017-2663-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2663-5