Abstract
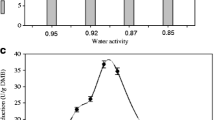
Solid-state fermentation using the microfungus Penicillium brevicompactum for the production of mycophenolic acid is reported in this paper. Of the initial substrates tested (whole wheat, cracked wheat, long grain Basmati rice, and short grain Parmal rice), Parmal rice proved to be the best. Under initial conditions, using steamed Parmal rice with 80% (w/w) initial moisture content, a maximum mycophenolic acid concentration of 3.4 g/kg substrate was achieved in 12 days of fermentation at 25 °C. The above substrate was supplemented with the following additional nutrients (g/L packed substrate): glucose 40.0, peptone 54.0, KH2PO4 8.0, MgSO4⋅7H2O 2.0, glycine 7.0, and methionine 1.65 (initial pH 5.0). A small amount of a specified trace element solution was also added. The final mycophenolic acid concentration was increased to nearly 4 g/kg substrate by replacing glucose with molasses. Replacing Parmal rice with rice bran as substrate further improved the mycophenolic acid production to nearly 4.5 g/kg substrate.





Similar content being viewed by others
References
Ismaiel, A. A., Ahmed, A. S., & El-Sayed, E.-S. R. (2014). Optimization of submerged fermentation conditions for immunosuppressant mycophenolic acid production by Penicillium roqueforti isolated from blue-molded cheeses: enhanced production by ultraviolet and gamma irradiation. World Journal of Microbiology and Biotechnology, 30(10), 2625–2638.
Ardestani, F., Fatemi, S. S. A., Yakhchali, B., Hosseyni, S. M., & Najafpour, G. (2010). Evaluation of mycophenolic acid production by Penicillium brevicompactum MUCL 19011 in batch and continuous submerged cultures. Biochemical Engineering Journal, 50(3), 99–103.
Xie, X., Jiang, Y., Lai, X., Xiang, S., Shou, Z., Chen, J., & Rizzari, M. (2015). mTOR inhibitor versus mycophenolic acid as the primary immunosuppression regime combined with calcineurin inhibitor for kidney transplant recipients: a meta-analysis. BMC Nephrology, 16(1), 91.
Yoo, C. G., Nghiem, N. P., Hicks, K. B., & Kim, T. H. (2013). Maximum production of fermentable sugars from barley straw using optimized soaking in aqueous ammonia (SAA) pretreatment. Applied Biochemistry and Biotechnology, 169(8), 2430–2441.
Abd Rahman, A. N., Tett, S. E., & Staatz, C. E. (2013). Clinical pharmacokinetics and pharmacodynamics of mycophenolate in patients with autoimmune disease. Clinical Pharmacokinetics, 52(5), 303–331.
Davidson, A., & Diamond, B. (2001). Autoimmune diseases. The New England Journal of Medicine, 345(5), 340–350.
De Winter, B. C. M., & Van Gelder, T. (2008). Therapeutic drug monitoring for mycophenolic acid in patients with autoimmune diseases. Nephrology, Dialysis, Transplantation, 1(3).
Séguin, V., Gente, S., Heutte, N., Vérité, P., Kientz-Bouchart, V., Sage, L., & Garon, D. (2014). First report of mycophenolic acid production by Eurotium repens isolated from agricultural and indoor environments. World Mycotoxin Journal, 7(3), 321–328.
Xu, Z. N., & Yang, S. T. (2007). Production of mycophenolic acid by Penicillium brevicompactum immobilized in a rotating fibrous-bed bioreactor. Enzyme and Microbial Technology, 40(4), 623–628.
Dong, Y., Xu, R., Wang, L., Zhang, J., Bai, C., Sun, A., & Wei, D. (2015). A combined feeding strategy for enhancing mycophenolic acid production by fed-batch fermentation in Penicillium brevicompactum. Process Biochemistry, 50(3), 336–340.
Alani, F., Grove, J. A., Anderson, W. A., & Moo-Young, M. (2009). Mycophenolic acid production in solid-state fermentation using a packed-bed bioreactor. Biochemical Engineering Journal, 44(2–3), 106–110.
Sadhukhan, A. K., Ramana Murthy, M. V., Ajaya Kumar, R., Mohan, E. V. S., Vandana, G., Bhar, C., & Venkateswara Rao, K. (1999). Optimization of mycophenolic acid production in solid state fermentation using response surface methodology. Journal of Industrial Microbiology & Biotechnology, 22, 33–38.
Nigam, P., & Singh, D. (1994). Solid-state (substrate) fermentation systems and their applications in biotechnology. Journal of Basic Microbiology, 34(6), 405–423.
Pandey, A., Soccol, C. R., & Mitchell, D. (2000). New developments in solid state fermentation: I-bioprocesses and products. Process Biochemistry, 35(10), 1153–1169.
Gombert, A. K., Pinto, A. L., Castilho, L. R., & Freire, D. M. G. (1999). Lipase production by Penicillium restrictum in solid-state fermentation using babassu oil cake as substrate. Process Biochemistry, 35(1–2), 85–90.
Raimbault, M. (1998). General and microbiological aspects of solid substrate fermentation. Electronic Journal of Biotechnology, 1(2), 174–188.
Muller dos Santos, M., Souza da Rosa, A., Dal’Boit, S., Mitchell, D. A., & Krieger, N. (2004). Thermal denaturation: is solid-state fermentation really a good technology for the production of enzymes? Bioresource Technology, 93(3), 261–268.
Dhillon, G. S., Brar, S. K., Verma, M., & Tyagi, R. D. (2011). Enhanced solid-state citric acid bio-production using apple pomace waste through surface response methodology. Journal of Applied Microbiology, 110(4), 1045–1055.
Chen, H. (2013). Introduction. In Modern solid state fermentation (pp. 1–21). Dordrecht: Springer Netherlands.
Angel-Cuapio, A., Figueroa-Montero, A., Favela-Torres, E., Viniegra-Gonzlez, G., Perraud-Gaime, I., & Loera, O. (2015). Critical values of porosity in rice cultures of Isaria fumosorosea by adding water hyacinth: effect on conidial yields and quality. Applied Biochemistry and Biotechnology, 177(2), 446–457.
Pandey, A., Ashakumary, L., Selvakumar, P., & Vijayalakshmi, K. S. (1994). Influence of water activity on growth and activity of Aspergillus niger for glycoamylase production in solid-state fermentation. World Journal of Microbiology & Biotechnology, 10(4), 485–486.
Bakri, Y., Jacques, P., & Thonart, P. (2003). Xylanase production by Penicillium canescens 10-10c in solid-state fermentation. Applied Biochemistry and Biotechnology, 108(1), 737–748.
Torrado, A. M., Cortés, S., Manuel Salgado, J., Max, B., Rodríguez, N., Bibbins, B. P., & Manuel Domínguez, J. (2011). Citric acid production from orange peel wastes by solid-state fermentation. Brazilian journal of microbiology: [publication of the Brazilian Society for Microbiology], 42(1), 394–409.
Chen, H. (2013). Aerobic solid-state fermentation. In Modern solid state fermentation (pp. 141–197). Dordrecht: Springer Netherlands.
Shaligram, N. S., Singh, S. K., Singhal, R. S., Pandey, A., & Szakacs, G. (2009). Compactin production studies using Penicillium brevicompactum under solid-state fermentation conditions. Applied Biochemistry and Biotechnology, 159(2), 505–520.
Viccini, G., Mannich, M., Maria, D., Capalbo, F., Valdebenito-Sanhueza, R., & Mitchell, D. A. (2007). Spore production in solid-state fermentation of rice by Clonostachys rosea, a biopesticide for gray mold of strawberries. Process Biochemistry, 42, 275–278.
Patel, G., Patil, M. D., Soni, S., Khobragade, T. P., Chisti, Y., & Banerjee, U. C. (2016). Production of mycophenolic acid by Penicillium brevicompactum—a comparison of two methods of optimization. Biotechnology Reports, 11, 77–85.
Prabhu, S., Sharma, P. D., Tiwari, S., Melarkode, R., Gururaja, R., & Suryanarayan, S. (2004). Process for producing mycophenolic acid using Penicillium arenicola BICC 7673. U.S. Patent no. 2008/0227164 A1.
Patil, M. D., Shinde, K. D., Patel, G., Chisti, Y., & Banerjee, U. C. (2016). Use of response surface method for maximizing the production of arginine deiminase by Pseudomonas putida. Biotechnology Reports, 10, 29–37.
Cai, Y., Liang, X., Liao, X., Ding, Y., Sun, J., & Li, X. (2010). High-yield hypocrellin a production in solid-state fermentation by Shiraia sp. SUPER-H168. Applied Biochemistry and Biotechnology, 160(8), 2275–2286.
Revankar, M. S., Desai, K. M., & Lele, S. S. (2007). Solid-state fermentation for enhanced production of laccase using indigenously isolated Ganoderma sp. Applied Biochemistry and Biotechnology, 143(1), 16–26.
Patil, M. D., Patel, G., Surywanshi, B., Shaikh, N., Garg, P., & Chisti, Y. (2016). Disruption of Pseudomonas putida by high pressure homogenization: a comparison of the predictive capacity of three process models for the efficient release of arginine deiminase. AMB Express, 6, 84.
El-Naggar, M. Y., El-Assar, S. A., & Abdul-Gawad, S. M. (2009). Solid-state fermentation for the production of meroparamycin by Streptomyces sp. strain MAR01. Journal of Microbiology and Biotechnology, 19(5), 468–473.
Hymery, N., Vasseur, V., Coton, M., Mounier, J., Jany, J. L., Barbier, G., & Coton, E. (2014). Filamentous fungi and mycotoxins in cheese: a review. Comprehensive Reviews in Food Science and Food Safety, 13(4), 437–456.
Niu, X., Qiu, S., Wu, Y., Yuan, J., & Xu, Y. (2011). Highly effective extraction of oil from soybean by pretreatment of solid-state fermentation with fungi. Frontiers of Chemical Science and Engineering, 5(1), 122–125.
De Castro, A. M., De Andréa, T. V., Dos Reis Castilho, L., & Freire, D. M. G. (2010). Use of mesophilic fungal amylases produced by solid-state fermentation in the cold hydrolysis of raw babassu cake starch. Applied Biochemistry and Biotechnology, 162(6), 1612–1625.
Silva, D., Tokuioshi, K., Martins, E. D. S., Da Silva, R., & Gomes, E. (2005). Production of pectinase by solid-state fermentation with Penicillium viridicatum RFC3. Process Biochemistry, 40(8), 2885–2889.
Aggelopoulos, T., Bekatorou, A., Pandey, A., Kanellaki, M., & Koutinas, A. A. (2013). Discarded oranges and brewer’s spent grains as promoting ingredients for microbial growth by submerged and solid state fermentation of agro-industrial waste mixtures. Applied Biochemistry and Biotechnology, 170(8), 1885–1895.
Veana, F., Martínez-Hernández, J. L., Aguilar, C. N., Rodríguez-Herrera, R., & Michelena, G. (2014). Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Brazilian journal of microbiology: [publication of the Brazilian Society for Microbiology], 45(2), 373–377.
Banat, I. M., Satpute, S. K., Cameotra, S. S., Patil, R., Nyayanit, N. V, & Spano, G. (2014). Cost effective technologies and renewable substrates for biosurfactants’ production. Frontier in Microbiology, 5, 1–81.
Awad, G. E. A., Helal, M. M. I., Danial, E. N., & Esawy, M. A. (2014). Optimization of phytase production by Penicillium purpurogenum GE1 under solid state fermentation by using Box-Behnken design. Saudi Journal of Biological Sciences, 21(1), 81–88.
Acknowledgements
GP and MP gratefully acknowledge the Department of Biotechnology (DBT), New Delhi, India for the award of Senior Research Fellowship. SS gratefully acknowledges the Department of Science and Technology (DST), New Delhi, India for the award of DST-INSPIRE fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Patel, G., Patil, M.D., Soni, S. et al. Production of Mycophenolic Acid by Penicillium brevicompactum Using Solid State Fermentation. Appl Biochem Biotechnol 182, 97–109 (2017). https://doi.org/10.1007/s12010-016-2313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2313-3