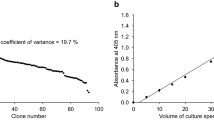
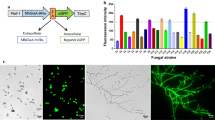
Abstract
The thermotolerant methylotrophic yeast Ogataea thermomethanolica is a host for heterologous protein expression via secretion to the culture medium. Efficient secretion is a major bottleneck for heterologous protein production in this strain. To improve protein secretion, we explored whether the use of a native signal peptide sequence for directing heterologous protein secretion and overexpression of native ER-resident chaperone genes could improve heterologous protein secretion in O. thermomethanolica. We cloned and characterized genes encoding α-mating factor (Otα-MF) and ER-resident chaperones OtBiP, OtCNE1, and OtPDI. The pre and pre-pro sequences of Otα-MF were shown to promote higher secretion of heterologous endoxylanase comparing with the classical pre-pro sequence of Saccharomyces cerevisiae. However, in the case of heterologous glycosylated phytase, only the Otα-MF pre-pro sequence significantly enhanced protein secretion. The effect of chaperone overexpression on heterologous protein secretion was tested in cotransformant cells of O. thermomethanolica. Overexpression of ER-resident chaperones improved protein secretion depending on heterologous protein. Overexpression of OtBiP, OtCNE1, and OtPDI significantly increased unglycosylated endoxylanase secretion at both 30 and 37 °C while only OtBiP overexpression enhanced glycosylated phytase secretion at 30 °C. These observations suggested the possibility to improve heterologous protein secretion in O. thermomethanolica.




Similar content being viewed by others
References
Celik, E., & Calık, P. (2012). Production of recombinant proteins by yeast cells. Biotechnology Advances, 30(5), 1108–1118.
Idiris, A., Tohda, H., Kumagai, H., & Takegawa, K. (2010). Engineering of protein secretion in yeast: strategies and impact on protein production. Applied Microbiology and Biotechnology, 86(2), 403–417.
Delic, M., Valli, M., Graf, A. B., Pfeffer, M., Mattanovich, D., & Gasser, B. (2013). The secretory pathway: exploring yeast diversity. FEMS Microbiology Reviews, 37(6), 872–914.
Yu, P., Zhu, Q., Chen, K., & Lv, X. (2015). Improving the secretory production of the heterologous protein in Pichia pastoris by focusing on protein folding. Applied Biochemistry and Biotechnology, 175(1), 535–548.
Hegde, R. S., & Bernstein, H. D. (2006). The surprising complexity of signal sequences. Trends in Biochemical Sciences, 31(10), 563–571.
Ahmad, M., Hirz, M., Pichler, H., & Schwab, H. (2014). Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Applied Microbiology and Biotechnology, 98(12), 5301–5317.
Jones, S. K., & Bennett, R. J. (2011). Fungal mating pheromones: choreographing the dating game. Fungal Genetics and Biology, 48(7), 668–676.
Kjeldsen, T., Hach, M., Balschmidt, P., Havelund, S., Pettersson, A. F., & Markussen, J. (1998). Prepro-leaders lacking N-linked glycosylation for secretory expression in the yeast Saccharomyces cerevisiae. Protein Expression and Purification, 14(3), 309–316.
Rakestraw, J. A., Sazinsky, S. L., Piatesi, A., Antipov, E., & Wittrup, K. D. (2009). Directed evolution of a secretory leader for the improved expression of heterologous proteins and full-length antibodies in Saccharomyces cerevisiae. Biotechnology and Bioengineering, 103(6), 1192–1201.
Lin-Cereghino, G. P., Stark, C. M., Kim, D., Chang, J., Shaheen, N., Poerwanto, H., & Lin-Cereghino, J. (2013). The effect of α-mating factor secretion signal mutations on recombinant protein expression in Pichia pastoris. Gene, 519(2), 311–317.
Yang, S., Kuang, Y., Li, H., Liu, Y., Hui, X., Li, P., & Wu, D. (2013). Enhanced production of recombinant secretory proteins in Pichia pastoris by optimizing Kex2 P1′ site. PLoS ONE, 8(9).
Lin, X. Q., Liang, S. L., Han, S. Y., Zheng, S. P., Ye, Y. R., & Lin, Y. (2013). Quantitative iTRAQ LC-MS/MS proteomics reveals the cellular response to heterologous protein overexpression and the regulation of HAC1 in Pichia pastoris. Journal of Proteomics, 91, 58–72.
Robinson, A. S., Hines, V., & Wittrup, K. D. (1994). Protein disulfide isomerase overexpression increases secretion of foreign proteins in Saccharomyces cerevisiae. Bio/Technology, 12(4), 381–384.
Harmsen, M. M., Bruyne, M. I., Raué, H. A., & Maat, J. (1996). Overexpression of binding protein and disruption of the PMR1 gene synergistically stimulate secretion of bovine prochymosin but not plant Thaumatin in yeast. Applied Microbiology and Biotechnology, 46(4), 365–370.
Zhang, W., Zhao, H. L., Xue, C., Xiong, X. H., Yao, X. Q., Li, X. Y., & Liu, Z. M. (2006). Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of Saccharomyces cerevisiae chaperone proteins. Biotechnology Progress, 22(4), 1090–1095.
Klabunde, J., Kleebank, S., Piontek, M., Hollenberg, C. P., Hellwig, S., & Degelmann, A. (2007). Increase of calnexin gene dosage boosts the secretion of heterologous proteins by Hansenula polymorpha. FEMS Yeast Research, 7, 1168–1180.
Qian, W., Liu, Y., Zhang, C., Niu, Z., Song, H., & Qiu, B. (2009). Expression of bovine follicle-stimulating hormone subunits in a Hansenula polymorpha expression system increases the secretion and bioactivity in vivo. Protein Expression and Purification, 68(2), 183–189.
Zhang, S. T., Fang, H. M., Zhao, L., Tian, Q. N., Qin, Y. F., Lu, P., & Liang, F. (2011). Co-overexpression of PpPDI enhances secretion of ancrod in Pichia pastoris. Applied Biochemistry and Biotechnology, 164(7), 1037–1047.
Limtong, S., Srisuk, N., Yongmanitchai, W., Yurimoto, H., & Nakase, T. (2008). Ogataea chonburiensis sp. nov. and Ogataea nakhonphanomensis sp. nov., thermotolerant, methylotrophic yeast species isolated in Thailand, and transfer of Pichia siamensis and Pichia thermomethanolica to the genus Ogataea. International Journal of Systematic and Evolutionary Microbiology, 58(1), 302–307.
Tanapongpipat, S., Promdonkoy, P., Watanabe, T., Tirasophon, W., Roongsawang, N., Chiba, Y., & Eurwilaichitr, L. (2012). Heterologous protein expression in Pichia thermomethanolica BCC16875, a thermotolerant methylotrophic yeast and characterization of N-linked glycosylation in secreted protein. FEMS Microbiology Letters, 334(2), 127–134.
Harnpicharnchai, P., Promdonkoy, P., Sae-Tang, K., Roongsawang, N., & Tanapongpipat, S. (2014). Use of the glyceraldehyde-3-phosphate dehydrogenase promoter from a thermotolerant yeast, Pichia thermomethanolica, for heterologous gene expression, especially at elevated temperature. Annals Microbiology, 64, 1457–1462.
Promdonkoy, P., Tirasophon, W., Roongsawang, N., Eurwilaichitr, L., & Tanapongpipat, S. (2014). Methanol-inducible promoter of thermotolerant methylotrophic yeast Ogataea thermomethanolica BCC16875 potential for production of heterologous protein at high temperatures. Current Microbiology, 69(2), 143–148.
Promdonkoy, P., Tang, K., Sornlake, W., Harnpicharnchai, P., Kobayashi, R. S., Ruanglek, V., & Tanapongpipat, S. (2009). Expression and characterization of Aspergillus thermostable phytases in Pichia pastoris. FEMS Microbiology Letters, 290(1), 18–24.
Ruanglek, V., Sriprang, R., Ratanaphan, N., Tirawongsaroj, P., Chantasigh, D., Tanapongpipat, S., & Eurwilaichitr, L. (2007). Cloning, expression, characterization, and high cell-density production of recombinant endo-1,4-xylanase from Aspergillus niger in Pichia pastoris. Enzyme and Microbial Technology, 41(1–2), 19–25.
Petersen, T. N., Brunak, S., von Heijne, G., & Nielsen, H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods, 8(10), 785–786.
Duckert, P., Brunak, S., & Blom, N. (2004). Prediction of proprotein convertase cleavage sites. Protein Engineering, Design and Selection, 17(1), 107–112.
Steentoft, C., Vakhrushev, S. Y., Joshi, H. J., Kong, Y., Vester-Christensen, M. B., Schjoldager, K. T.-B. G., & Clausen, H. (2013). Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. The EMBO Journal, 32(10), 1478–1488.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology, 23(3), 257–270.
Engelen, A. J., van der Heeft, F. C., Randsdorp, P. H., & Smit, E. L. (1994). Simple and rapid determination of phytase activity. Journal of AOAC International, 77(3), 760–764.
Zhang, T., Lei, J., Yang, H., Xu, K., Wang, R., & Zhang, Z. (2011). An improved method for whole protein extraction from yeast Saccharomyces cerevisiae. Yeast, 28(11), 795–798.
Bukau, B., & Horwich, A. L. (1998). The Hsp70 and Hsp60 chaperone machines. Cell, 92(3), 351–366.
Gupta, D., & Tuteja, N. (2011). Chaperones and foldases in endoplasmic reticulum stress signaling in plants. Plant Signaling & Behavior, 6(2), 232–236.
Darby, N. J., Kemmink, J., & Creighton, T. E. (1996). Identifying and characterizing a structural domain of protein disulfide isomerase. Biochemistry, 35(32), 10517–10528.
Ng, D. T. W., Brown, J. D., & Walter, P. (1996). Signal sequences specify the targeting route to the endoplasmic reticulum membrane. Journal of Cell Biology, 134(2), 269–278.
Fitzgerald, I., & Glick, B. S. (2014). Secretion of a foreign protein from budding yeasts is enhanced by cotranslational translocation and by suppression of vacuolar targeting. Microbial Cell Factory, 13(1), 125.
Ihara, Y., Cohen-Doyle, M. F., Saito, Y., & Williams, D. B. (1999). Calnexin discriminates between protein conformational states and functions as a molecular chaperone in vitro. Molecular Cell, 4(3), 331–341.
Danilczyk, U. G., & Williams, D. B. (2001). The lectin chaperone calnexin utilizes polypeptide-based interactions to associate with many of its substrates in vivo. Journal of Biological Chemistry, 276(27), 25532–25540.
Smith, J. D., Tang, B. C., & Robinson, A. S. (2004). Protein disulfide isomerase, but not binding protein, overexpression enhances secretion of a non-disulfide-bonded protein in yeast. Biotechnology and Bioengineering, 85(3), 340–350.
Robinson, A. S., Bockhaus, J. A., Voegler, A. C., & Wittrup, K. D. (1996). Reduction of BiP levels decreases heterologous protein secretion in Saccharomyces cerevisiae. Journal of Biological Chemistry, 271(17), 10017–10022.
Butz, J. A., Niebauer, R. T., & Robinson, A. S. (2003). Co-expression of molecular chaperones does not improve the heterologous expression of mammalian G-protein coupled receptor expression in yeast. Biotechnology and Bioengineering, 84(3), 292–304.
Van Der Heide, M., Hollenberg, C., Van Der Klei, I., & Veenhuis, M. (2002). Overproduction of BiP negatively affects the secretion of Aspergillus niger glucose oxidase by the yeast Hansenula polymorpha. Applied Microbiology and Biotechnology, 58(4), 487–494.
Maréchal, A., Tanguay, P.-L., Callejo, M., Guérin, R., Boileau, G., & Rokeach, L. A. (2004). Cell viability and secretion of active proteins in Schizosaccharomyces pombe do not require the chaperone function of calnexin. The Biochemical Journal, 380(Pt 2), 441–448.
Delic, M., Graf, A. B., Koellensperger, G., Haberhauer-Troyer, C., Hann, S., Mattanovich, D., & Gasser, B. (2014). Overexpression of the transcription factor Yap1 modifies intracellular redox conditions and enhances recombinant protein secretion. Microbial Cell, 1(11), 376–386.
Ruth, C., Buchetics, M., Vidimce, V., Kotz, D., Naschberger, S., Mattanovich, D., & Gasser, B. (2014). Pichia pastoris Aft1—a novel transcription factor, enhancing recombinant protein secretion. Microbial Cell Factory, 13, 120.
Gu, L., Zhang, J., Du, G., & Chen, J. (2015). Multivariate modular engineering of the protein secretory pathway for production of heterologous glucose oxidase in Pichia pastoris. Enzyme and Microbial Technology, 68, 33–42.
Acknowledgments
We are grateful to Dr. Philip J. Shaw for critically editing the manuscript. Financial support (P-12-01067) from National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency, Thailand is greatly appreciated. This work was also partially supported by Mahidol University. We thank Dr. Duangdao Wichadakul for Blastp analysis of O. thermomethanolica α-MF. S.K. is thankful for the scholarship from Thailand Graduate Institute of Science and Technology (TGIST). N.R. acknowledges the International Center for Biotechnology, Osaka University for invitation as a visiting research scholar.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 531 kb)
Rights and permissions
About this article
Cite this article
Roongsawang, N., Puseenam, A., Kitikhun, S. et al. A Novel Potential Signal Peptide Sequence and Overexpression of ER-Resident Chaperones Enhance Heterologous Protein Secretion in Thermotolerant Methylotrophic Yeast Ogataea thermomethanolica . Appl Biochem Biotechnol 178, 710–724 (2016). https://doi.org/10.1007/s12010-015-1904-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1904-8