Abstract
Deterioration of steel infrastructures is often caused by corrosive substances. In harsh conditions, the protection against corrosion is provided by high-performance coatings. The major challenge in this field is to find replacements for the fossil-based resins constituting anticorrosive coatings, due to increasing needs to synthesize new environmentally friendly materials. In this study, softwood Kraft lignin was epoxidized with the aim of obtaining a renewable resin for anticorrosive coatings. The reaction resulted in the formation of heterogeneous, solid, coarse agglomerates. Therefore, the synthetized lignin particles were mechanically ground and sieved to break up the agglomerates and obtain a fine powder. To reduce the use of fossil fuel-based epoxy novolac resins in commercial anticorrosive coatings, a series of formulations were prepared and cured on steel panels varying the content of epoxidized lignin resin. Epoxidized lignin-based coatings used in conjunction with conventional epoxy novolac resin demonstrated improved performance in terms of corrosion protection and adhesion properties, as measured by salt spray exposure and pull-off adhesion test, respectively. In addition, the importance of size fractionation for the homogeneity of the final coating formulations was highlighted. The findings from this study suggest a promising route to develop high-performing lignin-based anticorrosive coatings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term corrosion refers to the degradation of metals that occurs when they react with substances from the environment such as moisture and oxygen. This phenomenon is visible through the formation of rust and, if not mitigated, affects the performance of metals used in engineering and infrastructures. As a result, the corrosion protection market is one of the most important production sectors today. Several reports, including one from Global Market Insights,1 have shown that the market value of corrosion protection reached $13.5 billion in 2020 and will increase sharply in the coming years, with a forecast of $20 billion by 2027. Organic coatings represent the most commonly used method for corrosion protection of metallic materials and generally provide a protective barrier between the substrate and the environment with high resistance to ionic movement.2
Epoxy coatings account for most of the global value generated by the production of organic coatings. They are used to protect against corrosion in aggressive environments in equipment such as that used in oil and gas installations,3,4 and marine5 and energy applications.6 The base ingredients of epoxy coatings are epoxy resins, which combine excellent adhesion properties to metals with high resistance to harsh conditions such as heat, water, and certain chemicals.7 An epoxy resin and a curing agent, usually an amine compound, are mixed and a crosslinking reaction occurs, resulting in the formation of a thermoset polymer. Unparalleled corrosion protection is provided by the highly crosslinked molecular structure, which usually contains aromatic molecules. Good adhesion to substrates is also endured by the presence of pendant hydroxyl groups in the molecular structure.8 Considering all these properties, epoxy resins are often used as primers or intermediate coatings, on top of which a topcoat is usually applied.9 Most commercial epoxy coatings consist of bisphenol A (BPA) derivatives. The most commonly used for industrial applications is the diglycidyl ether bisphenol A (DGEBA). The aromatic segments of this molecule give the coatings high stiffness and good adhesion to the metal substrates.10 (BPA)-type epoxy coatings are usually combined with curing agents to form a binder formulation with high viscosity, which sometimes leads to difficulties in mixing.11 Therefore, alternatives such as bisphenol F (BPF)-type epoxy resins and phenol formaldehyde (n-PF) are commercially available. BPF-type epoxy resins have equivalent properties to the BPA types and also have lower viscosity, higher reactivity, and easy curing at ambient conditions.11 The n-PF resins, known commercially as epoxy novolacs, are used either alone or in combination with DGEBA.12 Apart from being based on fossil raw materials, environmental and human health concerns have been raised for materials made from bisphenol molecules.13,14,15
In order to have more sustainable corrosion protection systems, alternatives to the current fossil-based phenolic resins must be found while maintaining good coating performance. The most abundant natural resource for aromatic structures is lignin, a natural polymer that makes up 20% to 40% of wood tissue, depending on the species. Technical lignins are recovered as a side stream from pulping processes and are typically used for energy recovery. The amount of lignin produced worldwide is estimated at 50 to 70 million tons/year,16 but less is produced as refined lignin fractions. In recent years, new methods for separating lignin from the pulping process have been developed and used on a larger scale.17 It is therefore foreseeable that large quantities will be available in the near future.
Technical lignins are highly functional polymers containing phenolic, aliphatic, and carboxylic hydroxyl groups that can be chemically modified toward materials applications.18 Technical lignins differ significantly from native lignin. Their structure is highly dependent on the source and the method of separation. For example, technical Kraft lignin has a higher phenolic content than native lignin. The high functionality of technical lignins makes it an excellent candidate for chemical modifications to develop new bio-based materials.18 Technical lignins are mainly produced by a Kraft pulping process using sodium hydroxide (NaOH) and sodium sulfide (Na2S) to obtain black liquor as a product stream. Various processes can be used to separate lignin from the black liquor. One example is the LignoBoostTM process,17 which is based on the precipitation of lignin by changing the pH. Industrially, the separation of lignin from the Kraft process is rapidly growing compared to other extraction methods, such as lignosulfonates or organosolv.19
Several reaction pathways have been explored with the aim of exploiting the hydroxyl functionalities of Kraft lignin, such as acetylation,20 allylation,21,22 and silylation.23 Epoxidation of Kraft lignin has been investigated in previous studies after lignin fractionation24 or depolymerization,25 to obtain a more homogeneous system. This reaction is conventionally carried out by reactions of lignin hydroxyl groups with epichlorohydrin under alkaline conditions.26 Although functionalization of fractionated or depolymerized lignin has shown good results, scaling up to larger amounts still remains a challenge. To improve scalability, other epoxidation procedures have been carried out using technical Kraft27 or Organosolv lignin28 as retrieved, leading to promising results. In particular, the Organosolv lignin epoxidation approach developed by Over and co-workers28 has shown potentially scalable results. They reported a system containing DGEBA and 42 wt% epoxidized lignin showing higher glass transition temperature and better stiffness compared to a thermoset consisting of only DGEBA as resin.
Although the direct use of lignin is sometimes limited due to its high heterogeneity and polydispersity, which make it difficult to combine with conventional systems,29 a few studies have reported the incorporation of unmodified technical lignin in epoxy coatings as a substitute for pigments and fillers,30,31,32 showing good physical and mechanical properties. Laxminarayan et al.33 demonstrated a way to form homogeneous films by incorporating size-fractionated Kraft lignin particles, which were reduced to a suitable powder by mechanical milling and sieving. In this case, the introduction of unmodified lignin showed a significant improvement in adhesion performance compared to commercial coatings, as well as comparable long-term corrosion protection, after exposure for 70 days in a corrosive environment.
While the introduction of unmodified lignin has demonstrated the efficacy of a bio-based functional additive, the development of a scalable process to use epoxidized lignin as a resin component that is chemically crosslinked with curing agents is still an open research topic. In addition, there are no reliable tests in the literature showing the long-term corrosion resistance of coatings with incorporated lignin after epoxidation, and the results obtained are sometimes controversial. Previous electrochemical experiments conducted by Singh et al.34 with resins containing up to 20 wt% epoxidized lignin have shown poorer corrosion protection activity, while Komartin et al.35 have demonstrated promising performance in composites containing epoxidized linseed oil and Kraft lignin, resulting in a 140-380 % increase in corrosion protection.
The goal of the present work is twofold. One is to demonstrate a scalable route for the synthesis of epoxidized Kraft lignin (EKL) resins without further purification or fractionation processes; the other is to evaluate the properties of amine-cured epoxy novolacs when different amounts of EKL are used as resin component. Since epoxidized lignin particles are present in the coating matrix in particulate form, they could serve both as a resin and as substitute for pigments and additives. The present study demonstrates the possibility of scaling up a technique that would allow the use of epoxidized lignin as a bio-based component in engineering applications that typically require superior adhesion, corrosion, and mechanical resistance.
Experimental
Materials
Epoxidation of Kraft lignin was performed using the following compounds: softwood LignoBoostTM Kraft lignin (KL) kindly donated by StoraEnso (Finland); epichlorohydrin (ECH, ≥ 99%), potassium hydroxide (KOH, ≥ 90%), tetra-n-butylammonium bromide (TBAB, ≥ 98%), and n-hexane (≥ 99%) provided by Sigma-Aldrich; dichloromethane (DCM, ≥ 99.8%) provided by Supelco. Coatings formulations were prepared using the following chemicals: epoxy novolac resin DEN 438-X80 (EN); EPON 862 bisphenol F resin (DGEBF); pigment wet dispersant (WD) TroysperseTM CD1. The curing agent meta-xylenediamine 7% adduct (MXDA) was prepared by mixing meta-xylenediamine and bisphenol A (BPA) provided by Merck Life Science Aps (Denmark) and YTD-128 KUKDO Chemical Co. Ltd. (Korea), respectively. The curing agent cycloaliphatic amine hardener was supplied by Amicure PACM, Evonik Industries (Germany). All the other chemicals with analytical grade were received from Sigma-Aldrich.
Characterization analyses, particle modification, and performance evaluation
Phosphorus nuclear magnetic resonance spectroscopy (31P NMR)
Quantitative 31P NMR spectra were recorded at room temperature with a Bruker Advance III HD 400 MHz instrument with a BBFO probe and equipped with Z-gradient coil. All the data were analyzed by using MestReNova software V 14.2. This technique was utilized to obtain quantification of the different hydroxyl groups in the KL present before and after epoxidation, following the protocol developed by Argyropoulos in 1994.36 Accordingly, an internal standard (IS) solution was prepared by adding in 500 μL of pyridine, ≈30 mg N-hydroxy-5-norbornene−2,3-dicarboiamide (NHND, 97%), and ≈3.5 mg of chromium(III) acetylacetonate used as relaxation agent. Thereafter, accurately 30 mg of lignin was dissolved in a solution containing 100 μL of pyridine and 100 μL of dimethylformamide (DMF). After complete dissolution, 50 μL of IS and 100 µL of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP, 95%) were added. TMDP was used as reagent able to phosphorylate the hydroxyl functionalities of lignin, subsequently forming a precipitate. The whole solution was redissolved in 400 μL of deuterated chloroform (CDCl3). The spectra were acquired with an inverse-gated decoupling pulse (zgig30) with an acquisition time of 2 s, a relaxation delay of 10 s, and 128 number of scans. 1H NMR allowed the identification of epoxy functionalities. Then, ≈15 mg of lignin samples was dissolved in 600 µL of deuterated DMSO (DMSO-d6).
Attenuated total reflection–Fourier transform infrared spectroscopy (ATR–FTIR)
To analyze the degree of epoxidation of lignin specimens, FTIR analyses were performed using PerkinElmer 100 instrument in attenuated total reflection (ATR) mode with an MKII Golden Gate accessory (Specac Ltd.) equipped with a diamond crystal. FTIR analysis was also conducted to investigate the chemical structure of lignin-based epoxy coatings using a ThermoFisher Scientific Nicolet iS5 in ATR mode. Cured coatings were analyzed either directly as formed or after sanding of the surface. Measurements were obtained between 4000 and 600 cm−1, with a resolution of 4 cm−1 and 16 number of scans.
Differential scanning calorimetry (DSC)
DSC was performed to analyze the thermal transitions of the samples. The first heating cycle was conducted from room temperature up to 100 °C and then isothermally kept for 5 min. Subsequently, the samples were cooled to − 20 °C and heated to 400 °C. During the second heating, glass transition (Tg) was detected.
Dynamic mechanical analysis (DMA)
To analyze the viscoelastic properties, the coatings were cured as free films in silicon molds with a dimension of approximately 33 mm × 5.5 mm × 1.5 mm and clamped in a DMA Q800 equipped with pressurized air-cooling system. The samples were subjected to a strain of 0.1%, preload force of 0.1 N, and a force track of 125.0%. The samples were initially cooled down at 0 °C followed by isothermal for 5 min and then a ramping temperature of 3 °C/min to 180 °C. Sample specimens were either tested directly after ambient curing or after being post-cured at 100 °C in an oven for 2 h.
Scanning electron microscopy (SEM)
The observation of the cross section of lignin-based films was conducted on a field emission Hitachi SEM S-4800 microscope in order to investigate the internal structure of the materials. The images of the specimens were observed on a field emission Hitachi SEM S-4800 microscope. The images were captured at different magnifications (from 500 to 10000) and accelerating voltage (1.0, 1.2, or 1.6 kV) at a working distance of 8.5–9.5 mm. The cross sections were prepared by breaking the free films specimens using a scalpel. The cross sections of samples were placed on conductive carbon tape. All samples were sputtered with Pt/Pd system for 30 s, at a current of 80 mA with a Cressington 208HR sputter coater. The coating layer formed was about 2 nm thick, measured through quartz crystal microbalance.
Size fractionation of lignin
A Planetary Ball Mill PM 100 by Retsch was utilized in order to perform the grinding procedure of epoxidized lignin resin particles. The equipment consisted in a compact bench top single grinding station. Three steel balls mill (Ø30 mm) and approximately 25 g of lignin particles were put in the grinding jar which was successively clumped into the grinding station. Then, the milling procedure was started at 400 rpm for 10 min and repeated three times, obtaining a light brown fine powder. After grinding the particles, an analytical sieve shaker Retsch AS 200 control was used to sieve the powder to give a fine resin using space meshes of 63 µm. The sieving time was set at 15 min with an amplitude of 1.70 mm/gravity. After sieving, the particles with larger size than the meshes were collected and the grinding and sieving procedure was repeated to increase the overall yield until 95 wt%.
Laser diffraction particle size analysis
According to Mie scattering theory (ISO 13320-137 and ISO 9276-138), the size distribution of epoxidized lignin particles before and after size fractionation was measured using the laser diffraction MasterSizer 3000 from Malvern Technology Company. The particle and dispersant refractive index were defined, respectively, as 1.61 and 1.0. Ten repetitions for each sample were conducted in order to obtain an accurate standard deviation.
Pull-off adhesion
To test the adhesive resistance of coated surfaces, the pull-off test was performed according to EN ISO 4624:2016,39 utilizing an Elcometer model 510. Two aluminum dollies (Ø10 mm) were applied on the surface of the coatings using a formulated two-component BPF epoxy-amine adduct adhesive glue (similar to Araldite) and allowed to dry for more than 24 h. The surfaces were sanded prior to gluing of the dollies in order to increase the contact area. The dollies were pulled at a pull rate of 0.7 MPa/s, and the final force required to break the coating bond to the panel was measured.
Salt spray exposure
Salt spray test was performed to evaluate long-term corrosion resistance and to detect defects in coatings, according to EN ISO 9227-2022.40 A scribe of 50 mm × 2 mm was created in the cured film down to the steel substrate, using CNC milling machine. Then, according to the standard procedure, all the sides and the back of the substrates were coated with a commercial coating (Hempaline Defend 630). The tests were carried out in an enclosed, temperature-controlled tank where the samples were placed in specific supports. A 5 wt% sodium chloride solution was atomized from the nozzles, and the chamber was sealed and maintained at 35 ± 2 °C with a humidity level of 50 ± 1% for the entire duration of the tests.
Rust creep assessment
The rust creep was measured after 70 days of salt spray exposure, evaluating the thickness of delamination around the scribe of the coatings previously created. The coating around the scribe was removed by positioning a knife blade between the film and the substrate and applying a certain force manually. According to EN ISO 12944-9:2018,41 the rust creep value, M, was evaluated using the following equation
where C is the average of nine width measurements and W is the width of the scribe.
Pendulum hardness
Hardness of coatings was evaluated according to ISO 152242 by measuring the damping time of a König oscillating pendulum model Erichsen 299/300. The coated glass panels (DFT = 70 ± 5 µm) of dimension 100 mm × 150 mm × 6 mm were placed under the pendulum initially deflected to 6° and released, while a digital oscillation counter started at the same time. The number of oscillations of the pendulum was counted from 6° until it reached 3°. The number of oscillations was converted in seconds by multiplying the number of oscillations for 1.4 s.42
Direct impact resistance
The resistance, deformability, and ductility of the coatings to impacts were measured through impact tester Erichsen model 304. According to ISO 6272-1:2011,43 a ball (Ø20 mm/0.79″) of 1 kg was dropped freely down a guide tube with increasing height, until cracks were observed on the surface. The deformation of the surface after direct impacts was investigated by a digital microscope Keyence VHX-6000. The images were captured between 20.0x and 30.0x of magnification.
Synthesis and coatings preparation
Kraft lignin epoxidation
Raw KL (50 g–336 mmol OH) was suspended in excess of epichlorohydrin (ECH) (100 ml–1.28 mol) in a three-neck round-bottom flask equipped with a condenser and immersed in an ice bath. Then, TBAB (9 g–59.2 mmol) was added in portions and the mixture was blended for 1 h. KOH in the form of flakes (1.59 eq. the number of OH functionalities of lignin) was slowly added during vigorous mixing, over the course of 2.5 h. The reaction mixture was then stirred at room temperature for 5 h. The mixture was then diluted with DCM (300 ml) and the organic phase extracted with water (5 ml × 200 ml). The organic phase was then concentrated to roughly 100 ml by evaporating solvents with a rotary evaporator. Finally, the product was precipitated in n-hexane, decanted, and the residue dried in a vacuum oven for 24 h at 50 °C resulting in a hard-brown cake. The product (EKL) was manually ground in a mortar, redispersed in water, and agitated overnight to produce a coarse particle dispersion, which was then filtered and dried in a vacuum oven for additional 24 h. The amount of EKL obtained ranged between 40 and 55 g depending on losses during the experiment. EKL particles were then mechanically ground and sieved to size-fractionate the particles resin using a mesh size of 63 µm. Figure 1 depicts the difference between size-fractionated epoxidized Kraft lignin (SF-EKL) and nonsize-fractionated epoxidized Kraft lignin (NF-EKL). It is proposed that the SF-EKL appears less dark due to light-scattering effects.
Coatings preparation
The base components of coatings formulation, i.e., epoxy novolac/DGEBF resin, epoxidized lignin (S-EKL/N-EKL), and wetting–dispersing agent, were stirred using a high-speed disperser (DISPERMAT CV3-PLUS, VMA-Getzmann GmbH, Germany) at approximately 5000 rpm until a well-dispersed formulation was formed. The epoxidized lignin resin replaced exactly 0, 25, or 50 wt% of the traditional resin depending on formulation. Xylene was then added dropwise to achieve the ideal coating viscosity. Finally, the amine curing agent (MXDA-adduct for EN-based or Amicure for DGEBF-based coatings) was added to the formulations in the amounts indicated by the stoichiometric ratios (SR) in Table 1. The final coating formulations were applied to grit-blasted mild steel panels (75 mm × 150 mm × 3 mm) that had been industrially sandblasted to a cleanliness of Sa 2½ in accordance with ISO 8503-144 with a medium (G) surface roughness profile in accordance with ISO 8501-1.45 A steel bar applicator was used to apply all coats. The coated panels were cured at room temperature for 7 days. After curing, the dry film thickness (DFT) was 300 ± 10 µm, accurately measured using a coating thickness gauge (Elcometer 355). The list of coating formulations is presented in Table 1. In Table 2 are schematized the molecular structures of the components utilized in coating formulations.
Results and discussion
Resin synthesis
Epoxidation of KL with epichlorohydrin utilizing TBAB and KOH catalysts was initiated in an ice bath (Scheme 1). The presence of the ice bath at the beginning of the reaction was required to maintain the temperature of this exothermic process below 50 °C, in order to avoid risk of side reactions.28,46,47
The EEW of EKL was estimated by comparison of the 31P NMR before and after epoxidation, as well as considering the additional molecular weight of the epoxy groups (Fig. 2). All of the phenolic and carboxylic hydroxyls reacted completely. Some aliphatic alcohol groups remained unreacted, and a new signal was detected between 145 and 146.5 ppm. The presence of the novel signal was previously explored and determined to be the result of either NMR-phosphite derivatization of the phenolic-linked epoxide or from incomplete synthesis of the oxirane ring after epoxidation.24
Indeed, the phenolic and carboxylic acid hydroxyl groups totally reacted, decreasing from 3.8 and 0.4 to 0 mmol g−1, respectively. Because phenolic and carboxylic acid hydroxyls are known to react with epoxides, removing these groups completely ensures that the EKL can only chemically react with the amine crosslinker. If the remaining acid or phenolic functionalities are not eliminated, they may influence the stoichiometric ratio of epoxides and amines, making the data more difficult to interpret.
A summary of the quantification of functional groups before and after epoxidation is shown in Table 3. The value of EEW of EKL was calculated according to the results obtained from the evaluation of 31P NMR.
Comparison of the FTIR spectra in Fig. 3 showed more evidence of lignin epoxidation. Stretching vibrations of hydroxyls functionalities between 3700 and 3100 cm−1 are greatly reduced after epoxidation, validating previous findings from 31P NMR spectra. The epoxidation additionally increases the vibration signal of the C–H bonding vibration signal between 3100 and 2750 cm−1. The existence of C–O–glycidyl (ether) is shown at 1089 cm−1, and the new signals at 908 and 755 cm−1 are indicative of C–O–C oxirane asymmetric stretching vibration. These findings are consistent with previous studies on lignin epoxidation.25,28,48,49 According to the FTIR data, the new peak in the 31P NMR spectrum resulted from an interaction between an epoxide and the phosphorylating agent.
The particle size distribution was determined using a laser diffraction particle size analyzer. As indicated in Fig. 4, the particle size of the sieved and ground particles was less that of the nonsieved. The average particle size of SF-EKL was 51 µm, while that of NF-EKL was 139 µm. When assuming a smooth spherical particle shape, the specific surface area of NF-EKL was determined to be 74.3 m2 kg−1. The surface area increased to 1148 m2 kg−1 after grinding and sieving operations. This result indicates that the contact area between the SF-EKL and the binders was 15 times higher than for NF-EKL, which contributed significantly to the coatings’ ultimate homogeneity and performance. Figure S16 depicts particle patterns and shapes as determined by SEM analyses.
Coatings formulations and film formation
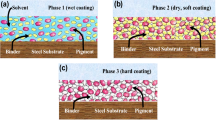
A high-speed disperser was used to create a variety of coating compositions. The epoxidized lignin was produced as a particulate solid resin that was xylene insoluble. As a result, the coating compositions were made up of a mix of conventional epoxy resins, hardener, xylene, and dispersed epoxidized lignin resin particles. Curing and drying at room temperature allowed for resemble procedures commonly employed in industry. This, however, raises the possibility of vitrification and post-curing consequences as shown in the thermal properties paragraph. Except for the coating containing NF-EKL, which had a rough agglomerated topography, all of the dried and cured films had a smooth surface, as illustrated in Fig. 6. According to scheme 2, the curing process was the result of a combination between physical drying by solvent evaporation and a chemical crosslinking.
The FTIR spectra of MXDA-adduct, SF-EKL, and the sanded surfaces of Coatings (a), (e), and (g) are shown in Fig. 5a. The spectra comparing the cured films with 25 and 50 wt% SF-EKL [Coatings (e), (g)] to the reference panel [Coating (a)] show no significant differences. The signal relating to N–H bending vibration at 3342 cm−1, as shown in Fig. 5a, b, sharply reduces after curing, as does the intensity of the signal linked to C–O–C oxirane ring stretching in all coatings at 904 cm−1. This confirms a proper stoichiometric equilibrium between epoxy resins and curing agent with SR of 0.75. Figure 5b shows unreacted MXDA from the N–H bending vibration signal at 3342 cm−1 in the nonsanded surface of Coating (f) with an SR of 0.90. Unreacted MXDA caused amine flushing, resulting in a visible waxy film on the coated surface. Amine blushing is not just a physical defect, but it can also impair adhesive and barrier qualities.50 As demonstrated in Figure S1, there was no significant difference in the FTIR spectra of the panels containing NF-EKL and SF-EKL. Figure S2 shows the same pattern for formulations employing DGEBF as a base resin, but with a slightly higher number of residual epoxides.
Figure 6 shows the appearance of the coatings prior to being subjected in the spray chamber for anticorrosion testing, as well as the scribes required for testing. The hue of the SF-EKL coatings ranged from a light brown (SF-EKL 25 wt%) to a dark brown (SF-EKL 50 wt%). Furthermore, the difference in homogeneity between by SF-EKL and NF-EKL coatings is obvious due to agglomerates generated by the big particles, as observed in our previous research on unmodified lignin particles33. Table 1 is used to recover the composition of all coatings.
Appearance of all samples prior to salt spray exposure test. The compositions of the coatings are summarized in Table 1
Thermal and mechanical properties
DSC and DMA were used to assess the thermal and mechanical properties of coatings. During the first heating cycle, the DSC measurements revealed post-curing events above 50 °C (Fig. S5). This demonstrates the existence of vitrification when the films were cured at room temperature. The Tg measurements during the second heating cycle (Figs. S3, S4) indicated only one transition for all samples, showing that the cured coatings had homogeneous characteristics. It has previously been demonstrated on technical Kraft lignin thermosets utilizing thiol–ene chemistry that, while the lignin resin is homogeneously dispersed inside the thermoset, the lignin introduces nanometer-scale molecular ordering.51 The addition of lignin resin resulted in a larger transition as well as a lower Tg with increasing lignin resin content. Previously, broad glass transitions were seen on various Kraft lignin-based thermosets.22 The Tgs ranged from 80 to 95 °C for films having 25 wt% SF-EKL or NF-EKL [Coatings (e), (f), (d)] and the reference films [Coatings (a), (b)]. The Tg, on the other hand, fell to values between 70 and 75 °C in formulations containing 50% lignin resin. Variations in curing agent quantities did not result in statistically significant variations. DMA was used to gain further thermal characteristics, as illustrated in Figs. S6–S12. The presence of post-curing effects was investigated in samples containing just EN, in particular in Coating (a). The Tg computed by observing the peak of the tan δ was lower than the value acquired through DSC experiments. Figure S9 indicates that the storage modulus (E') above Tg increases with temperature. The presence of a post-curing phase, beginning at an onset temperature of 75 °C, can explain this phenomenon. To test the viscoelastic properties after post-curing, another sample from the same formulation was heated to 100 °C for 2 h.
Further thermal properties were obtained by performing DMA, as shown in Figs. S6–S12. The presence of post-curing effects was studied on the samples containing only EN, in particular of Coating (a). The Tg, calculated by observing the peak of the tan δ, was lower than the value previously obtained from the DSC tests. The storage modulus (E’) above Tg shows an increase with temperature, as shown in Fig. S9. The reason for this behavior can be explained by the presence of post-curing process, starting from an onset value of 75 °C. Therefore, to investigate the viscoelastic properties after post-curing, another sample from the same formulation was placed in an oven for 2 h at 100 °C. In this situation, the Tg value jumped from 60 to 102 °C, although E' did not rebound significantly. This type of viscoelastic behavior in epoxy-amine systems can be predicted in general because post-curing is routinely used in the polymer sector, particularly in novolac resins, to enhance the Tg.52
DSC measurements of DGEBF coatings revealed less broad and higher transition values, with temperatures ranging from 95 to 100 °C. All of the preceding findings and results for EN systems were present in DGEBF amine-cured systems, as illustrated in Figs. S8andS11. Table 4 displays all Tgs collected before and after post-curing.
Coating performance
Adhesion
The results of pull-off adhesion tests given in Table 5 reveal that coatings composed of EN resins have superior adhesion qualities than DGEBF resins. The presence of SF-EKL in combination with EN considerably improved adhesion performance. Cured coatings having 25 wt% of SF-EKL [Coatings (e) and (f)] had the highest pull-off resistance (21.0 and 20.1 MPa). The inclusion of 50 wt% of lignin [Coatings (g) and (i)] increased adherence compared to pure novolac. When the same amount of lignin but a larger amount of curing agent (SR of 0.90) was added to the coating mixture, the adhesion strength decreased. The particle size of the epoxy lignin had a significant impact on the adhesion qualities. Because of the physical inhomogeneities caused by the NF-EKL resin in the cured films, a rather low adhesion strength (9 MPa) with a considerable standard deviation was found. In comparison with novolacs, DGEBF-based coatings had comparatively low adherence. The inclusion of SF-EKL resin in the formulations enhanced adhesion of DGEBF coating, but only marginally. Coatings appearance after pull-off adhesion tests is shown in Fig. S13. Similar patterns were observed in prior research, where unmodified lignin was incorporated in the coating formulations, yielding a pull-off adhesion strength of 23 ± 0.5 MPa.33 The type of failures derived from the pull-off adhesion tests by evaluating the dolly–coating interfaces are displayed in Table 5 and Figure S13. The dolly–glue failure without any adhesive or cohesive failure, in Coatings (e) and (f), demonstrates the excellent adhesion performance of the coatings containing 25 wt% of SF-EKL with SR = 0.75.
Impact force
The results of the direct impact resistance test on coated panels are summarized in Table 5 in order to evaluate the resistance to cracking. From the results, it is evident that the presence of SF-EKL in Coating (e) (shown in Fig. 7), and Coating (g) improved the impact resistance. Indeed, they withstand an impact force up to 0.38 and 0.35 N, respectively, whereas the neat EN [Coating (h)] with the same the SR showed an impact strength of 0.25 N. Increasing SR to 0.90, the impact resistance dramatically decreased to 0.15 N for Coating (f) and 0.25 N for Coating (g), if compared to the neat EN [Coating (b)] showing an impact resistance of 0.37 N. Then, it is evident that in some cases part of the curing agent can remain unreacted, affecting negatively the impact resistance. Coating (d) (containing NF-EKL) exhibited a worse impact strength compared to Coating (e) (containing SF-EKL), with the same SR and wt% of lignin. Furthermore, as demonstrated in the previous study, the presence of nonsize-fractionated unmodified Kraft lignin in epoxy novolac33 and DGEBA53-based coatings decreased the impact strength, whereas the size-fractionated particles gave superior impact resistance.33 DGEBF-based coatings [Coating (h) and Coating (c)] showed a low impact resistance, and in this case, the presence of SF-EKL did not improve the impact performance.
Hardness
The results from the hardness test are presented in Table 5, while an example of coated glass substrate for the test is presented in Fig. S14. The highest number of oscillations (around 160) was obtained for DGEBF-based coatings, corresponding to the hardest surface. A similar result was achieved in our previous study for DGEBF-based coatings where it was supposed that the cycloaliphatic amine curing agent used (Amicure) in these systems forms a highly crosslinked network, bringing it to a more brittle but harder film.33 The inhomogeneous surface of Coating (d) containing both NF-EKL and EN gave a significant drop of about 38% in the number of oscillations compared to Coating (e) containing both SF-EKL and EN [Coating (e)] and Coating (a) containing only EN, demonstrating the importance of considering the particle size of EKL. In conclusion, the use of SF-EKL in EN-based coatings did not show any variation on the hardness properties. Similar results of the hardness performances were achieved in reference (33) after unmodified Kraft lignin incorporation.
Anticorrosion performance
The coatings’ corrosion resistance was assessed by exposing the samples to a salt spray chamber. The coatings after 70 days of exposure are shown in Fig. 8. On neat EN and neat DGEBF, blisters and pitting corrosion were detected (Fig. 8a, b, c). These corrosion areas were probably caused by pinholes formed during coating application as well as a lack of pigments, which are widely used in industrial manufacturing. Pinhole defects in the NF-EKL-based coating (Fig. 8d) indicated that nonhomogeneous films, due to the presence of coarse particles, negatively affect the anticorrosion properties. The coatings containing EN together with SF-EKL (Fig. 8e, f, g) worked flawlessly, with no surface defects identified when 25% or 50 wt% of SF-EKL was added to the formulation, confirming its superior barrier protection over NF-EKL. However, the presence of SF-EKL in DGEBF panels (Fig. 8h) does not necessarily increase the long-term corrosion protection properties. In this case, water entry during the test generated inhomogeneities and pitting damages. A key factor could be the difference in chemical structure between SF-EKL and DGEBA compared to EN, which could result in lesser interaction between the molecules, and as a result, a less crosslinked network, allowing water to easily enter. Coating (i) (see Table 1) cured too rapidly during coating application, due to the high concentration of solid resin SF-EKL and curing agent, resulting in voids on the coating. As a result, this formulation was deemed inconsistent and insignificant for anticorrosion tests. Also encouraging results were achieved in the previous study33 where surface flaws were not identified for coatings containing size-fractionated unmodified lignin particles.
Anticorrosion properties after 70 days (1680 h) in salt spray exposure chamber of coatings. The compositions of the coatings are summarized in Table 5
Rust creep measurements
Rust creep was measured after 70 days of exposure in a salt spray chamber to see how far the corrosion traveled along the substrate. In Fig. 9, examples of rust creep evaluation are presented for three panels.
Examples of rust creep measurements after 70 days (1680 h) on Coatings (a), (f), and (g). The compositions of the coatings are summarized in Table 4
As demonstrated in Table 6, the EN Coating (e) with 25 wt% of SF-EKL and SR of 0.75 (2.20 mm) had the lowest rust creep. This result outperforms even average commercial coatings, which showed rust creep of 2.98 mm after 70-day exposure as determined in a recent study.33 However, a larger concentration of SF-EKL had no positive effect on the rust creep formation. Indeed, it can be noticed that at 50 wt% of SF-EKL in Coating (g) a higher creep (3.48 mm) was attained. These findings indicate that establishing the right combination of SF-EKL and amine during formulation is both difficult and critical. The DGEBF-SF-EKL Coating (h) had the worst creep value (3.95 mm), which was even higher than the neat DGEBF Coating (c) (3.44 mm). The anticorrosion properties and rust creep formation suggested that EN and SF-EKL coatings are more compatible than DGEBF and SF-EKL coatings. When compared to coatings containing size-fractionated lignin, EN-NF-EKL Coating (d) had a higher rust creep value (2.81 mm). The unseen voids formed by massive agglomerates, as well-established in our previous research33 and in Fig. 8d in our study, could provide an access point for corrosive species, causing more substantial rust creep.
Conclusions
Technical Kraft lignin was successfully epoxidized in the current work to yield a solid epoxy resin (EKL). The lignin-based epoxy resin was size fractionated (SF-EKL) and employed in anticorrosive coating formulations with traditional epoxy resins. As a benchmark, a formulation using nonsize-fractionated epoxidized lignin (NF-EKL) was developed. In each of the formulations, the amount of epoxidized lignin was precisely 0, 25, or 50 wt% of the conventional resin, i.e., epoxy novolac (EN) or diglycidyl ether bisphenol F (DGEBF).
All samples composed of EN and SF-EKL showed no surface defects after 70 days of salt spray exposure, while the reference coating (pure EN) showed slight defects. The coating composed of EN and NF-EKL exhibited pitting defects after 70 days, showing the influence of particle size on the probability of defect formation. For all samples composed of DGEBF, some blisters and pitting defects were formed after 70 days, and the presence of SF-EKL did not give significant improvement in this case. The coating with 25 wt% of SF-EKL and a SR of 0.75 showed the highest rust creep resistance (2.20 mm).
The results of the adhesion tests corroborate well with the results of the corrosion tests, as the formulations with EN and SF-EKL showed a considerable improvement from around 13.5 MPa to roughly 20.5 MPa of pull-off resistance. Additionally, it was discovered that the impact resistance of the coatings was greatly increased by having an ideal stoichiometric ratio and SF-EKL concentration. The hardness performances did not show any improvement after the introduction of SF-EKL. Thermal properties showed that post-curing effects exist when coatings are first cured at ambient conditions observing that Tg derived from the tan δ increased from 60 to 100 °C after heating films in an oven. The presence of one Tg is significant since it implies that coatings are fairly homogeneous after curing.
In conclusion, the results demonstrated that the anticorrosive and adhesion properties of epoxy novolac coating were significantly improved by the addition of size-fractionated epoxidized Kraft lignin resin particles.
The petroleum-based epoxy resins can thus be partially replaced by these resins, opening the door to a new bio-based industrial product. The next obvious step is to upscale the binder synthesis from gram to kilo scale and beyond, with a critical consideration of the energy costs involved.
References
Pulidindi, K. and Mukherjee, S, https://www.gminsights.com/industry-analysis/anti-corrosion-coatings-market. Global Market Insights, 2021.
Lyon, SB, Bingham, R, Mills, DJ, “Advances in Corrosion Protection by Organic Coatings: What We Know and What We Would Like to Know.” Prog. Org. Coat., 102 2–7. https://doi.org/10.1016/j.porgcoat.2016.04.030 (2017)
Rajagopalan, N, et al. “Degradation Mechanisms of Amine-Cured Epoxy Novolac and Bisphenol F Resins under Conditions of High Pressures and High Temperatures.” Prog. Org. Coat., 156 106268. https://doi.org/10.1016/j.porgcoat.2021.106268 (2021)
Rajagopalan, N, et al. “Influence of CO2 at HPHT Conditions on the Properties and Failures of an Amine-Cured Epoxy Novolac Coating.” Ind. Eng. Chem. Res., 60 (41) 14768–14778. https://doi.org/10.1021/acs.iecr.1c02713 (2021)
Atta, AM, et al. “New Bisphenol Novolac Epoxy Resins for Marine Primer Steel Coating Applications.” Prog. Org. Coat., 63 (4) 372–376. https://doi.org/10.1016/j.porgcoat.2008.06.013 (2008)
Lee, J-R, et al. “A Study on Physicochemical Properties of Epoxy Coating System for Nuclear Power Plants.” Nucl. Eng. Des., 236 (9) 931–937. https://doi.org/10.1016/j.nucengdes.2005.09.017 (2006)
Sørensen, PA, et al. “Anticorrosive Coatings: A Review.” J. Coat. Technol. Res., 6 (2) 135–176. https://doi.org/10.1007/s11998-008-9144-2 (2009)
Rouw, AC, “Model Epoxy Powder Coatings and Their Adhesion to Steel.” Prog. Org. Coat., 34 181–192 (1998)
Weiss, KD, “Paint and Coatings: A Mature Industry in Transition.” Prog. Polym. Sci., 22 203–245 (1997)
Ng, F, et al. “Bio-Based Aromatic Epoxy Monomers for Thermoset Materials.” Molecules, 22 (1) 149. https://doi.org/10.3390/molecules22010149 (2017)
Jin, NJ, et al. “Effects of Curing Temperature and Hardener Type on the Mechanical Properties of Bisphenol F-Type Epoxy Resin Concrete.” Constr. Build. Mater., 156 933–943. https://doi.org/10.1016/j.conbuildmat.2017.09.053 (2017)
Mathew, D, Reghunadhan Nair, CP, Ninan, KN, “Bisphenol A Dicyanate-Novolac Epoxy Blend Cure Characteristics, Physical and Mechanical Properties, and Application in Composites.” J. Appl. Polym. Sci., 74 1675–1685. https://doi.org/10.5028/jatm.2010.02027610 (1999)
Le, HH, et al. “Bisphenol A Is Released from Polycarbonate Drinking Bottles and Mimics the Neurotoxic Actions of Estrogen in Developing Cerebellar Neurons.” Toxicol. Lett., 176 (2) 149–156. https://doi.org/10.1016/j.toxlet.2007.11.001 (2008)
Moon, MK, “Concern about the Safety of Bisphenol A Substitutes.” Diabetes Metab. J., 43 (1) 46–48. https://doi.org/10.4093/dmj.2019.0027 (2019)
Vandenberg, LN, et al. “Human Exposure to Bisphenol A (BPA).” Reprod. Toxicol., 24 (2) 139–177. https://doi.org/10.1016/j.reprotox.2007.07.010 (2007)
Luo, H, Abu-Omar, MM, “Lignin Extraction and Catalytic Upgrading from Genetically Modified Poplar.” Green Chem., 20 (3) 745–753. https://doi.org/10.1039/c7gc03417b (2018)
Tomani, P, “The Lignoboost Process.” Cellul. Chem. Technol., 44 53–58 (2010)
Laurichesse, S, Avérous, L, “Chemical Modification of Lignins: Towards Biobased Polymers.” Prog. Polym. Sci., 39 (7) 1266–1290. https://doi.org/10.1016/j.progpolymsci.2013.11.004 (2014)
Dessbesell, L, et al. “Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers.” Renew. Sustain. Energy Rev., 123 109768. https://doi.org/10.1016/j.rser.2020.109768 (2020)
Jeong, H, et al. “Use of Acetylated Softwood Kraft Lignin as Filler in Synthetic Polymers.” Fibers Polym., 13 (10) 1310–1318. https://doi.org/10.1007/s12221-012-1310-6 (2012)
Ribca, I, et al. “Exploring the Effects of Different Cross-Linkers on Lignin-Based Thermoset Properties and Morphologies.” ACS Sustain. Chem. Eng., 9 (4) 1692–1702. https://doi.org/10.1021/acssuschemeng.0c07580 (2021)
Jawerth, M, et al. “Renewable Thiol-Ene Thermosets Based on Refined and Selectively Allylated Industrial Lignin.” ACS Sustain. Chem. Eng., 5 (11) 10918–10925. https://doi.org/10.1021/acssuschemeng.7b02822 (2017)
Buono, P, et al. “New Insights on the Chemical Modification of Lignin: Acetylation Versus Silylation.” ACS Sustain. Chem. Eng., 4 (10) 5212–5222. https://doi.org/10.1021/acssuschemeng.6b00903 (2016)
Gioia, C, et al. “Tunable Thermosetting Epoxies Based on Fractionated and Well-Characterized Lignins.” J. Am. Chem. Soc., 140 (11) 4054–4061. https://doi.org/10.1021/jacs.7b13620 (2018)
Ferdosian, F, et al. “Sustainable Lignin-Based Epoxy Resins Cured with Aromatic and Aliphatic Amine Curing Agents: Curing Kinetics and Thermal Properties.” Thermochim. Acta, 618 48–55. https://doi.org/10.1016/j.tca.2015.09.012 (2015)
Nikafshar, S, et al. “Choosing the Right Lignin to Fully Replace Bisphenol A in Epoxy Resin Formulation.” ChemSusChem, 14 (4) 1184–1195. https://doi.org/10.1002/cssc.202002729 (2021)
Mansouri, N-EE, Yuan, Q, Huang, F, “Synthesis and Characterization of Kraft Lignin Based Epoxy Resins.” BioResources, 6 2492–2503 (2011)
Over, LC, et al. “Synthesis and Characterization of Epoxy Thermosetting Polymers from Glycidylated Organosolv Lignin and Bisphenol A.” Macromol. Chem. Phys., 218 (4) 1600411. https://doi.org/10.1002/macp.201600411 (2017)
Duval, A, et al. “Solvent Screening for the Fractionation of Industrial Kraft Lignin.” Holzforschung, 70 (1) 11–20. https://doi.org/10.1515/hf-2014-0346 (2016)
Nonaka, Y, Tomita, B, Hatano, Y, “Synthesis of Lignin/Epox Resins in Aqueous Systems and Their Properties.” Holzforschung, 51 183–187. https://doi.org/10.1515/hfsg.1997.51.2.183 (1997)
Simionescu, CI, et al. “Lignin/Epoxy Composites.” Compos. Sci. Technol., 48 317–323. https://doi.org/10.1016/0266-3538(93)90149-B (1994)
Feldman, D, et al. “Structure–Properties Relations of Thermally Cured Epoxy–Lignin Polyblends.” J. Appl. Polym. Sci., 42 (6) 1537–1550. https://doi.org/10.1002/app.1991.070420607 (1991)
Laxminarayan, T, et al. “Chemically-Resistant Epoxy Novolac Coatings: Effects of Size-Fractionated Technical Kraft Lignin Particles as a Structure-Reinforcing Component.” Prog. Org. Coat., 183 107793. https://doi.org/10.1016/j.porgcoat.2023.107793 (2023)
Singh, A, Yadav, K, Kumar Sen, A, “Sal (Shorea Robusta) Leaves Lignin Epoxidation and Its Use in Epoxy Based Coatings.” Am. J. Polym. Sci., 2 (1) 14–18. https://doi.org/10.5923/j.ajps.20120201.03 (2012)
Komartin, RS, et al. “Composite Materials from Renewable Resources as Sustainable Corrosion Protection Coatings.” Polymers, 13 (21) 3792. https://doi.org/10.3390/polym13213792 (2021)
Argyropoulos, D, “Quantitative Phosphorus-31 NMR Analysis of Six Soluble Lignins.” J. Wood Chem. Technol., 14 (1) 65–82. https://doi.org/10.1080/02773819408003086 (1994)
ISO 13320-1: Particles size analysis-laser diffraction methods. International Organization for Standardization, Switzerland, 2020.
ISO 9276-1: Representation of results of particle size analysis–Part 1: Graphical representation. International Organization for Standardization, Switzerland, 1998.
ISO 4624: Paints and varnishes–Pull-off test for adhesion. International Organization for Standardization, Switzerland, 2016.
ISO 9227: Corrosion tests in artificial atmospheres–Salt spray tests. International Organization for Standardization, Switzerland, 2022.
ISO 12944-9: Paints and varnishes–Corrosion protection of steel structures by protective paint systems– Part 9: Protective paint systems and laboratory performance test methods for offshore and related structures. International Organization for Standardization, Switzerland, 2018.
ISO 1522: Paints and varnishes–Pendulum damping test. International Organization for Standardization, Switzerland, 2006.
ISO 6272-1: Paints and varnishes—Rapid-deformation (impact resistance) tests—Part 1: Falling-weight test, large-area indenter. International Organization for Standardization, Switzerland, 2011.
ISO 8503-4: Preparation of steel substrates before application of paints and related products–surface roughness characteristics of blast-cleaned steel substrates–Part 4: Method for the calibration of ISO surface profile comparators and for the determination of surface profile–Stylus instrument procedure. International Organization for Standardization, Switzerland, 2012.
ISO 8501-1, Preparation of steel substrates before application of paints and related products—Visual assessment of surface cleanliness. International Organization for Standardization, Switzerland, 2007.
Malutan, T, Nicu, R, Popa, VI, “Lignin Modification by Epoxidation.” BioResources, 3 (4) 1371–1376 (2008)
Over, LC, Meier, MAR, “Sustainable Allylation of Organosolv Lignin with Diallyl Carbonate and Detailed Structural Characterization of Modified Lignin.” Green Chem., 18 (1) 197–207. https://doi.org/10.1039/c5gc01882j (2016)
Gioia, C, et al. “Lignin-Based Epoxy Resins: Unravelling the Relationship between Structure and Material Properties.” Biomacromolecules, 21 (5) 1920–1928. https://doi.org/10.1021/acs.biomac.0c00057 (2020)
Feng, P, Chen, F, “Lignin-Based Epoxy Blends.” BioResources, 7 (3) 2860–2870 (2012)
Mills, GD, “Amine Blush in Epoxy Coatings.” Mater. Performance. Coat & Linings, 52–55 (2008).
Jawerth, ME, et al. “Mechanical and Morphological Properties of Lignin-Based Thermosets.” ACS Appl. Polym. Mater., 2 (2) 668–676. https://doi.org/10.1021/acsapm.9b01007 (2020)
Rudd, RM, Ghafarian, SR, Taherkhani, A, “The Effects of Post Curing Process on the Physical Properties of Glass Fiber/Phenol—Formaldehyde Molded Composites.” J. Basic Appl. Sci. Res., 3 66–69 (2013)
Gouveia, JR, et al. “Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive.” Molecules, 25 (11) 2513. https://doi.org/10.3390/molecules25112513 (2020)
Acknowledgments
The authors would like to express their gratitude to the Knut and Alice Wallenberg Foundation through the Wallenberg Wood Science Center (WWSC) at KTH Royal Institute of Technology, the Nordic Five Tech Alliance, and The Hempel Foundation to CoaST (The Hempel Foundation Coatings Science and Technology Centre) for financial support.
Funding
Open access funding provided by Royal Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was presented at the 18th Coatings Science International Conference held on June 26–29, 2023, in Noordwijk, the Netherlands.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Truncali, A., Laxminarayan, T., Rajagopalan, N. et al. Epoxidized technical Kraft lignin as a particulate resin component for high-performance anticorrosive coatings. J Coat Technol Res (2024). https://doi.org/10.1007/s11998-023-00899-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11998-023-00899-9