Abstract
The aim of this work was to develop an innovative tomato cold soup fortified in bioactive compounds through the incorporation of UV-B–treated radish seeds. After a 20 kJ m−2 UV-B treatment, radish seeds increased their sulforaphene content by 30%. Different concentrations of UV-B–treated seeds (0, 0.5, 1.5, 3, and 5 g kg−1) were added to a chopped vegetables cold soup, mainly made of Kumato® cherry tomatoes as novelty, including pepper, cucumber, and garlic, which was stored for 8 days at 4 °C. Added seeds did not affect physicochemical quality attributes, microbial growth, nor sensory perception. Nevertheless, a dose-dependent behaviour was shown in glucoraphenin and sulforaphene content, according to concentrations of UV-B–treated seeds added. It was also appreciated after an in vitro digestion that the bioaccessible fraction of glucosinolates and isothiocyanates was kept constant throughout the refrigerated storage. The sulforaphene content of the soup increased by ~ 19% after 2 days at 4 °C, of which the 33% was bioaccessible (measured in vitro), and subsequently was degraded by ~ 20% after 8 days at 4 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum) is one of the most consumed horticultural products, with more than 180 million tonnes produced in 2020 which is presumed to be annually increased (FAO, 2022). In fact, due to the development of biotechnology and food technology, several varieties of tomatoes have been widely traded in our supermarkets, such as Kumato®. This cultivar is characterized by its colour, ranging from a green to reddish brown or purple, and its sweeter flavour due to its higher fructose content (Opara et al., 2012). Kumato® tomatoes are grown by specialized producers mainly located in Spain and other southern and central European countries, Australia, Mexico, Turkey, Lebanon, and Canada.
Moreover, tomato-based or tomato-derived food products are widely consumed all over the world. In particular, a cold tomato soup known as ‘gazpacho’ is a traditional ready-to-drink cold soup in the Spanish cuisine which has become also popular in other countries. It is made of chopped fresh raw vegetables such as tomato, garlic, pepper, and cucumber resulting in a convenient healthy choice to increase the consumption of horticultural commodities. Initially, gazpacho consumption was associated to homemade food, which was mainly eaten in summer, but currently, it is one of the most sold and consumed cold soups by the Spanish population (AESAN, 2019), independent of the location and the season. Moreover, these kinds of products have crossed borders and are increasingly consumed on the European continent, especially in the Mediterranean area.
From the first research works developed by Martinez-Valverde et al. (1999) to recent findings (González et al., 2019; Planes-Muñoz et al., 2021; Ströhla et al., 2022), gazpacho has been studied for being very rich in nutraceuticals with potential health benefits as antioxidants. In addition, Campra et al. (2019) showed potential in vitro anti-proliferative activity of cold tomato soups, as well as gazpacho consumption has been associated with an important reduction in the cardiovascular risk by diminishing blood pressure and hypertension in a cohort study of 3995 subjects (Medina-Remón et al., 2013). This behaviour was related to the richness of bioactive compounds present in the vegetable ingredients used to make the traditional recipe (tomato, cucumber, green pepper, onion, garlic, extra virgin olive oil, vinegar, and salt).
Nowadays, as a new generation of functional foods has emerged, a whole range of products derived from fruit and vegetables is being developed (Zhu et al., 2021). Tomato juice or puree has been previously used as a model to be enriched in anthocyanins with the addition of strawberries (Lafarga et al., 2019) or antioxidant dietary fibre with grape skins (Lavelli et al., 2015). In this sense, new vegetable-based formulations exogenously enriched in nutraceuticals from natural ingredients, such as smoothies, hummus, cold soups, and sauces, have constituted a very attractive new field of research for the food industry. For instance, the concentration of glucoraphanin in kale pesto sauce has been increased after the inclusion of a revalorized product obtained from Bimi broccoli leaves and mustard seeds in its formula, without affecting its sensory properties (Castillejo et al., 2021). In fact, the incorporation of mustard seeds aimed for glucoraphanin conversion to sulforaphane, as main bioactive compound. Radish (Raphanus sativus), and especially radish seeds, is an important source of glucosinolates and isothiocyanates (Martínez-Zamora et al., 2021a; Raiola et al., 2018; Soundararajan & Kim, 2018). Isothiocyanates, and mainly sulforaphene as the major compound found in radish, which differs from sulforaphane by the presence of a double bond, are known by their demonstrated anti-cancer properties due to their positive role acting as anti-inflammatory compounds, modulating cell cycles, and allowing the apoptosis of neoplastic cells (Raiola et al., 2018; Soundararajan & Kim, 2018). In fact, Brassica species have been already used, as a source of sulforaphane, for the improvement of beverages. Alvarez-Jubete et al. (2014) elaborated soups using dry soup mixes enriched with broccoli floret and stalk freeze-dried powders, which resulted in veggie soups rich in phytochemicals. Azarashkan et al. (2022) developed a broccoli sprout nanoliposomes extract with basil seed gum, without applying into any beverage, although they showed the bioaccessibility of such sulforaphane; hence, it could be applied to food fortification and pharmaceutical industries. In this sense, the application of such phytochemicals has gained interest in the last years, and its application can be made according to several methodologies.
Parallelly, and as a novelty of this work, the use of abiotic stressors to enhance the biosynthesis of phytochemicals can be used to enrich these ingredients. Specifically, UV-B applied at low doses has demonstrated to stimulate the photoreceptors and the genetic chain involved in the production of secondary metabolites (Jansen & Bornman, 2012; Jenkins, 2009; Schreiner et al., 2014) as glucosinolates and isothiocyanates in broccoli and radish sprouts (Martínez-Zamora et al., 2021b), and even when applied directly to seeds has shown its remaining effect on mustard sprouts (Martínez-Zamora et al., 2022).
Therefore, the objective of this study was to initially increase the glucosinolate and sulforaphene content in radish seeds by using UV-B as abiotic stressor, and afterwards, to develop an innovative cold tomato soup fortified in bioactive compounds throughout the incorporation of such UV-B–treated seeds and evaluating their in vitro bioaccessibility, evaluating the sensory profile and physicochemical quality while preserving its safety.
Material and Methods
Glucosinolates and Isothiocyanates Source
Radish (Raphanus sativus) seeds provided by Intersemillas S.A. (Valencia, Spain) were used as a natural source of glucosinolates and isothiocyanates (Martínez-Zamora et al., 2021b). Secondary metabolite production in radish seeds was endogenously stimulated by controlled exposure to 20 kJ m−2 UV-B in a chamber equipped with six unfiltered UV-B emitting lamps (TL 40W/01 RS; Philips, Eindhoven, The Netherlands) fully described in Martínez-Zamora et al. (2021b). Such a dose was selected according to our previous experiments and obtained results of glucosinolates and sulforaphene content of UV-B–treated and untreated radish seeds are shown in the section “Radish Seed Characterization”. One day after the UV treatment, treated seeds were ground using an electric grinder (Taurus Group, Oliana, Spain) (particle size < 4 µm) and immediately added to the cold tomato soup formulas.
Cold Tomato Soup Elaboration
Kumato® cherry tomatoes (Solanum lycopersicum), variety Syngenta seeds -KM5512- (Torre-Pacheco, Murcia, Spain), were harvested and supplied by G’s España (Torre-Pacheco, Murcia, Spain). The remaining ingredients of the tomato cold soup were purchased at a local supermarket.
The preparation of cold tomato soup was carried out in a disinfected cold room at 8 °C according to Verde Méndez et al. (2011) with slight modifications. The list of ingredients used for each formula is presented in Table 1, CTRL being the control sample and S0.5, S1.5, S3, and S5 the different formulas with the gradual doses of treated radish seeds.
Fresh vegetables were carefully inspected, selected, sanitized with 250 mg L−1 peracetic acid during 2 min, and then rinsed with cold water for 1 min. Five different formulas of cold tomato soup were prepared in a food processor (Robot Cook®, Robot Coupe; Vincennes, Île-de-France, France) used for chopping and mixing materials at room temperature. The supplementation with radish seeds and the use of cherry tomatoes instead of reddish mature tomatoes were the main novelty of this gazpacho formulation. According to the traditional gazpacho recipe, tomato represented 72% of the elaborated formula. After rapidly cooling down to 4 °C, each cold tomato soup was divided into 50-g tubes under aseptic conditions and stored for 8 days at 4 °C in darkness.
Six replicates per treatment and sampling day were prepared (5 formulations of cold tomato soups × 4 sampling days—0, 2, 5, and 8 days— × 6 replicates = 120 samples). Three samples of each formula were taken on each sampling day for physicochemical and microbial analyses, while another three samples per treatment were stored at –80 °C until further analysis of the bioactive compound content. The experimental design is presented in Fig. 1.
Physicochemical Quality Attributes Analysis
Physicochemical analyses were carried out in triplicate for each cold tomato soup formula and sampling day. Colour was determined using the CIELab system (L*, a*, and b* coordinates) equipped with an adapter for liquids, and colour differences (ΔE) throughout refrigerated storage were calculated according to Martínez-Zamora et al. (2021a). The total soluble solids content (TSS), pH, and titratable acidity (TA) were also determined following the methods described by Martínez-Zamora et al. (2021a).
Sensory Analysis
Sensory analyses were performed in a standard room by 12 trained panelists (ISO-8586:2012, 2012) on each sampling day of the experiment. Cold tomato soup samples were served in transparent glasses coded with three random digits. Still water and bread sticks were used among samples. Trained panelists evaluated from 1 (extremely bad) to 5 (excellent) the visual appearance, colour, aroma, flavour, and texture of reformulated tomato cold soups.
Microbial Analysis
Microbial growth (mesophilic, psychrophilic, enterobacteria, moulds, and yeasts) was analysed following the standard methods (Castillejo et al., 2016). Briefly, 10 mL of tomato cold soup was mixed with 90 mL of peptone water and subsequent dilutions (101–103 range) were made before surface sowing in petri dishes, in which PCA medium was used for mesophilic and psychrophilic, VRBD for Enterobacteriaceae, and Rose Bengal agar for moulds and yeasts. All microbial counts were reported as log colony forming units per gramme of product (log CFU g−1). Three replicates and three dilutions per treatment were analysed on each sampling day.
Nutraceutical Content Analysis
Glucosinolates
Extraction and analysis of glucosinolate content were carried out following the method described by Martínez-Zamora et al. (2021b). For that, freeze-dried samples of tomato cold soup (0.25 ± 0.01 g) were mixed with 10 mL of ethanol/water (70:30, v/v) previously heated at 70 °C in a bath. Samples were incubated at 70 °C for 30 min and vortexed every 10 min to ensure the enzymatic inactivation. Extracted samples were rapidly cooled on an ice bath and centrifuged at 18,000 g for 10 min at 4 °C. In total, 3 mL of the supernatant was used for purification of glucoraphenin on disposable polypropylene columns (Thermo Fisher Scientific, Waltham, MA, USA) previously conditioned (0.5 mL MilliQ water + 0.5 mL Sephadex A-25 + 0.5 mL MilliQ water). After washing with 1 mL MilliQ water + 1 mL of 0.02 M sodium acetate, glucoraphenin desulfation was carried out by adding 75 µL of purified sulfatase. Desulfo-glucoraphenin was eluted with 1.25 mL of MilliQ water and analysed in an ultra-high–performance liquid chromatography (UHPLC) instrument (Shimadzu, Kyoto, Japan) equipped with a DGU-20A degasser, LC-30AD quaternary pump, SIL-30AC autosampler, CTO-10AS column heater, and SPDM-20A photodiode array detector following the method described by Martínez-Zamora et al. (2021b). Desulfo-glucoraphenin was detected at 227 nm, and glucoraphanin (PhytoLab GmbH & Co. KG, Vestenbergsgreuth, Germany) was used as the external standard. The chromatogram of identified compounds in radish seeds is shown in Fig. S1, while the chromatogram of glucoraphenin in tomato cold soups is identified in Fig. S2. Data were expressed as gramme per kilogramme of fresh weight (fw). Each sample was extracted and analysed in triplicate.
Sulforaphene
Sulforaphene content was analysed according to Martínez-Zamora et al. (2021b). Briefly, 0.25 g of freeze-dried samples of tomato cold soup was mixed with 5 mL of MilliQ water (pH = 6) and incubated for 2 h at 45 °C. After cooling the samples, 25 mL MTBE was added. The mixture was sonicated for 1 min and dewatered using 5 g of Na2SO4 and filtered through Whatman 41. Extracted samples were purified on activated (3 mL MTBE + 3 mL ethyl acetate + 3 mL air) SI-1 silica cartridge (Phenomenex, Torrance, CA, USA). Sulforaphene was eluted with 2 mL methanol, evaporated in a rotary evaporator at 45 °C, and resuspended in 1 mL acetonitrile.
An UHPLC (see section above) was used as previously described by Martínez-Zamora et al. (2021b). Sulforaphene was quantified using its homologue DL-sulforaphane as the external standard (Sigma-Aldrich, St. Louis, MO, USA), and results were expressed as milligramme per kilogramme fw. Each sample was analysed in triplicate. The chromatogram of sulforaphene in radish seeds is shown in Fig. S1, while the chromatogram of tomato cold soups is shown in Fig. S3.
In Vitro Nutraceutical Bioaccessibility
In vitro nutraceutical bioaccessibility was evaluated by glucoraphenin (see section above) and sulforaphene (see section above) analysis after an in vitro digestion performed at 37 °C following the method previously described by Martínez et al. (2018). Briefly, 8.0 ± 0.5 g samples were homogenized with 40 mL MilliQ water and 0.7 mL HCl 6 N. Samples were incubated in darkness for 2 h at 37 °C after addition of 0.37 mL pepsin solution (2.5 g pepsin + 5 mL HCl 1 N + 45 mL MilliQ water), simulating a gastric digestion. After adjusting pH to 5.3, 1.85 mL pancreatic solution (7.5 g pancreatin + 1.512 g NaHCO3 + 90 mL MilliQ water) was added, and samples were incubated in darkness for 3 h at 37 °C, simulating an intestinal digestion. To stop the digestion procedure, digested samples were heated at 100 °C for 15 min, rapidly cooled in ice, and filtered.
Statistical Analysis
The experiment was a two-factor (radish seed dose × storage time) design subjected to analysis of variance (ANOVA) using Statgraphics Plus software (v. 5.1. Statpoint Technologies. Inc. Warrenton, VA, USA). Statistical significance was assessed at the level p < 0.05, and Tukey’s multiple range test was used to separate means. SigmaPlot 10.0 was used to create the artwork.
Results and Discussion
Radish Seed Characterization
Glucosinolates and sulforaphene, as main phytochemicals of radish seeds, were initially analysed before their addition as a functional ingredient in the tomato cold soup. Obtained results are shown in Table 2. Glucoraphenin was the main glucosinolate found in radish seeds, which represented 70% of the total glucosinolate content (also shown in Fig. S1), followed by other minor glucosinolate compounds, such as neoglucobrassicin, 4-hydroxy-glucobrassicin, glucoraphasatin, progoitrin, glucoiberin, glucobrassicin, and 4-methoxy-glucobrassicin. This identification can be comparable to previous results reported by Wang et al. (2022), who compared the glucosinolate profile of 111 varieties of radish taproots and seeds showing as the major compound found in radish seeds was glucoraphanin, which during plant growth decreased its concentration while increased the biosynthesis of glucoraphasatin, the major compound found in radish plants and taproots. After a 20 kJ m−2 UV-B treatment, glucoraphenin content was increased by 5%, stimulating subsequently the biosynthesis of sulforaphene by 28%, while glucobrassicin content suffered the highest increase (18%) after UV-B treatment. However, the other individual glucosinolates remained constant after UV-B treatment and the increase in total glucosinolate content (4%) was accounted for by glucoraphanin-dsg as the major glucosinolate.
These results agree with our recent findings, where 15 kJ m−2 UV-B stimulated the biosynthesis of glucoraphenin by ~ 30% and sulforaphene by ~ 72% in 10-day radish sprouts (Martínez-Zamora et al., 2021b). Nevertheless, the sulforaphene content in radish seeds is fourfold lower than in 10-day radish sprouts. So, previous experiments have been carried out to select the proper UV-B dose able to stimulate the sulforaphene biosynthesis in seeds before sprouting. It was corroborated that Epithiospecifier protein (ESP) activity must be lower in comparison with other Brassicaceae species (Martínez-Zamora et al., 2021b; Wang et al., 2017); hence, the great part of glucoraphenin is transformed to the bioactive form of sulforaphene by the action of the myrosinase enzyme. However, the action of myrosinase is not only responsible to produce isothiocyanates from native glucosinolates, but there is also a specific chemical rearrangement which, in the relative absence of epithiospecific proteins, leads to isothiocyanates instead of nitriles and epithionitriles. This may also vary depending on species, variety, and even the part of the plant evaluated. Previous authors have also shown the higher activity of this enzyme in radish (Yi et al., 2016), which makes UV-B–treated radish seeds a better source of sulforaphene instead of other seeds from the Brassicaceae family. These increases are the result of the activation of UV-B photoreceptors in plant organs, which are called UV-LOCUS 8 and are responsible for stimulating transcriptional factors that trigger secondary metabolism, such as the biosynthesis of glucosinolates and isothiocyanates (Artés-Hernández et al., 2022).
Physicochemical and Microbial Growth Changes of the Cold Tomato Soup
All gazpacho formulations showed a similar colour, being L* 43.92 ± 1.83, a* 2.22 ± 0.69, b* 16.14 ± 1.49, C* 16.30 ± 1.52, and hue° 82.22 ± 2.14 (Fig. 2), visually perceived in the samples (Fig. 1). Such colour was stable during the refrigerated storage period (Fig. 2). In fact, the average colour variation was uniform (ΔE < 5) throughout the shelf-life with no differences among treatments, nor sampling day. The incorporation of UV-B–treated radish seeds did not affect the colour perception of the tomato cold soups.
Colour changes of fresh Kumato® cherry tomato cold soup for 8 days at 4 °C. Presented colour coordinates are obtained as a mean of all treatments and sampling days. CTRL, control sample; S0.5, S1.5, S3, and S5 are the different formulas with the gradual doses (g kg-1) of UV-B–treated radish seeds. Different letters indicate significant differences (p < 0.05) among sampling days
In this sense, the traditional recipe of Spanish gazpacho usually has a more reddish colour, characterized by higher a* values (between 15 and 22) and b* (between 25 and 40), provided by the mature red tomatoes used for the elaboration (Pinilla et al., 2005). Nevertheless, given the new technologies and recipes frequently developed in the industry and cuisine, the gazpacho made with Kumato® cherry tomatoes presented in this study can be a novel ‘gourmet’ product with increased acceptability.
Due to the incorporation of Kumato®, a* and b* values of this recipe have shown a decrease in comparison with the traditional recipe described by Pinilla et al. (2005). For that reason, our gazpacho recipe shows a pale greenish orange colour due to the skin of tomatoes used, as main ingredient of the formulation, which reported a high visual acceptability according to a sensory analysis (Fig. 3).
This behaviour is also similar when referring to physicochemical parameters of reformulated tomato cold soups, whose values of pH, TSS, and TA were not affected by the incorporation of radish seeds (Table 3). All samples had a pH between 3.9 and 4.1, an average TSS of 7.38 ± 0.19%, and an average TA of 7.07 ± 0.26 g citric acid L−1. However, a slight positive tendence can be observed between TSS and radish seed concentration. In this sense, S3 and S5 treatments showed an increase of TSS in comparison with CTRL and S0.5, while S1.5 is sited in a mean value of all of them.
The obtained values agree with previous results of Pinilla et al. (2005), who obtained a similar pH, TSS, and TA of a traditional pasteurized tomato cold soup commercialized in Spain. Also, Elez-Martínez and Martín-Belloso (2007) and Verde Méndez et al. (2011) showed values stablished in the same range for such parameters, due to the fact that the traditional recipe of Spanish gazpacho follows the same proportions of the main ingredients (tomato, green pepper, cucumber, onion, garlic, olive oil, vinegar, and salt).
Due to the low pH of the tomato cold soup (between 3.9 and 4.1), bacteria growth did not exceed ~ 2–3 CFU g−1 during processing, nor after the refrigerated storage. In fact, such low pH, due to the high citric acid content of tomatoes, and to the incorporation of vinegar into the recipe, can avoid the microbial growth, which is optimum between pH of 6 and 8.5.
Mesophilic bacterial load at the beginning of the shelf-life was of 1 CFU g−1, which increased to ~ 2.25 CFU g−1 after 2 days at 4 °C and reach its maximum after 5 days with ~ 3 CFU g−1 (Table 4). Psychrophilic bacterial load was ~ 2 CFU g−1, which was kept constant during the shelf-life study, without remarkable differences among treatments (Table 4).
By contrast, significant differences were found in enterobacteria in the last days of storage (Table 4). After 5 and 8 days at 4 °C, reductions of 0.5 CFU g−1 were observed in S3 and S5 tomato cold soups. Similarly, on the 2nd day moulds and yeast growth also experimented a reduction produced by the high doses of radish seeds (S1.5, S3, and S5), wherein the sulforaphene content may have been influenced (Table 4). Sulforaphene identified from radish seeds has demonstrated to possess antimicrobial properties against some resistant bacteria, such as Helicobacter pylori at 0.6 mg L−1 and Staphylococcus aureus at 12.5–25.0 mg L−1 (Lim et al., 2016), which may explain the slight reduction in the microbial growth of our tomato cold soup, considering that we proportionally used a lower dose of UV-B–treated radish seeds.
Furthermore, as physicochemical quality parameters and microbiological growth were not negatively affected by radish seed incorporation, no differences were appreciated regarding the sensory perception of the reformulated tomato cold soups throughout the refrigerated shelf-life study (Table 4). In this sense, after 8 days at 4 °C, only a decrease of 0.5 points in the sensory scale was observed regarding the colour-turbidity, which can be related to the slight increase of ΔE on this day (Fig. 2), although this value is not relevant as it does not exceed the commercial limit.
Nutraceutical Content in Tomato Cold Soup and Its Bioaccessibility
Obtained chromatograms from glucosinolate analysis are shown in Fig. S2. Glucoraphenin, which was retained at 6.2 min and absorbed at 227 nm (Fig. S2.A), progressively increased its content according to the radish seed amount used to elaborate the cold soups, from CTRL to S5. Its content in CTRL samples after processing was null (Fig. 4), which increased to ~ 300 mg kg−1 fw in the rest of the treatments (S0.5, S1.5, S3, and S5). Slight increases by ~ 16 and ~ 32% are shown for S3 and S5 treatments after 2 days at 4 °C, respectively. The proposed hypothesis is that it could be due to the crushing stress radish seeds suffered before their addition to the cold soups. This behaviour can be justified by previous findings in shredded carrots, which also has shown an increase in bioactive compounds after cutting as an abiotic stress (Formica-Oliveira, Martínez-Hernández, Aguayo et al., 2017, Formica-Oliveira, Martínez-Hernández, Díaz-López et al., 2017; Jacobo-Velázquez & Cisneros-Zevallos, 2012).
Glucoraphenin content in fresh Kumato® cherry tomato cold soup and in vitro bioaccessibility. CTRL, control sample; S0.5, S1.5, S3, and S5 are the different formulas with the gradual doses (g kg−1) of UV-B–treated radish seeds. ***Significant differences p < 0.001. All reformulated tomato cold soups were significantly different (p < 0.001) to CTRL. Δ indicates significant differences regarding S0.5 treatment
Furthermore, a slight dose-dependence tendence can be observed during the refrigerated storage, which is also remarkable after in vitro digestion. S5 without prior digestion, followed by S3, showed the highest glucoraphenin content during the refrigerated storage (330 and 310 mg kg−1 fw, respectively), while S0.5 and S1.5 samples reported 270 and 280 mg kg−1 fw. After in vitro digestion, bioaccessibility of glucoraphenin was 80 mg kg−1 fw for S0.5, S1.5, and S3, while it was slightly increased by ~ 12.5% for S5 (Figs. S2 and 4). Therefore, the bioaccessibility and initial glucoraphenin content, as the main glucosinolate, was increased after the addition of radish seeds in the tomato cold soup.
However, the glucoraphenin content in UV-B radish seeds was 200,145 mg kg−1 fw (data shown in Table 2 in g kg−1 fw); hence, the concentration added in 5 g of radish seeds (S5) was 1000.7 mg kg−1 fw. This fact could be due to the action of the myrosinase enzyme and the degradation of glucoraphenin into sulforaphene, which is also corroborated after studying the sulforaphene concentration (Figs. 5 and 6).
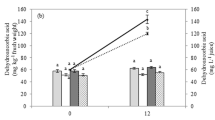
Sulforaphene content in fresh Kumato® cherry tomato cold soup and in vitro bioaccessibility. CTRL, control sample; S0.5, S1.5, S3, and S5 are the different formulas with the gradual doses (g kg−1) of UV-B–treated radish seeds. ***Significant differences p < 0.001. All reformulated tomato cold soups were significantly different (p < 0.001) to CTRL. Δ indicates significant differences regarding S0.5 treatment
In fact, the increase of bioactive compounds after the addition of radish seeds is more evident after studying the sulforaphene content in such samples (Figs. S3.A and 5). As the sulforaphene content in CTRL was null, just the addition of 0.5 g kg−1 fw radish seeds (S0.5) significantly increased its content to 1.57 mg kg−1 fw after processing, whose values were kept constant throughout shelf-life from 0.82 mg kg−1 fw after 2 days to 1.45 mg kg−1 fw after 8 days at 5 °C. In this case, sulforaphene content showed a strong dose-dependence tendence, which is due to its biosynthesis from glucoraphenin during a refrigerated storage. In fact, the sulforaphene content in S5 samples reached 47.22 mg kg−1 fw after 5 days at 4 °C, from which the 33% may be potentially bioaccessible to the human body (Figs. S3.B and 5).
Furthermore, the sulforaphene content in radish seeds was 2361 mg kg−1 fw (data shown in Table 2 in g kg−1 fw), which means that 12 mg sulforaphene was added to S5 cold soup samples. Nevertheless, S5 showed almost 45 mg sulforaphene kg−1 fw after processing, which corroborates that this compound has been synthetized not only during processing from glucoraphenin by the action of myrosinase, but also throughout the refrigerated storage (Fig. 6). Although 33% of the sulforaphene consumed in the reformulated cold soups was potentially in vitro bioaccessible, more than the total of the sulforaphene added from radish seeds (12 mg kg−1 fw) was highly bioaccessible after an in vitro digestion (13 mg kg−1 fw), thanks to the action of the myrosinase enzyme that led the conversion from glucoraphenin to sulforaphene. This behaviour can be explained by the fact that the optimal conditions for the action of this enzyme are at 37 °C and neutral pH (Parchem et al., 2020), conditions in which the intestinal phase of in vitro digestion was performed.
Among Brassica vegetables, which are rich in carotenoids, vitamin C, fibre, flavonoids, and glucosinolates, radish seeds are the richest one in sulforaphene content, obtained from the glucosinolate hydrolysis catalysed by myrosinase. As widely known, sulforaphene from Raphanus sativus seeds has been used as an anti-inflammatory and anticancer compound in Korean traditional medicine (Kim et al., 2014). As a stomachal protector, radish seeds have shown to be potential agents to avoid flatulence, liquid and mucus retention, constipation, and gastric reflux (Kim et al., 2014). Sulforaphane is also known as a regulator of the genetic materials that counteract the oxidative stress causing inflammation and DNA damage, as the main precursor of tumour development. Specifically, the inhibiting function of sulforaphane is to avoid the metabolism of the enzymes that convert procarcinogen agents to carcinogen ones, whose action can also be suppressed by sulforaphane provoking the apoptosis of tumoural cells (Clarke et al., 2008; Liang et al., 2019). In this sense, sulforaphenes have shown to have similar biological activities and effects on cancer cells (Cheng et al., 2020; Gao et al., 2021).
In this way, consumption of 200 µM (35 mg sulforaphane) per day for healthy consumers is recommended by Egner et al. (2014) and Riedl et al. (2009), while Lozanovski et al. (2014, 2020) recommended a daily intake of 500 µM (87 mg sulforaphene) to improve the chemotherapy treatment in pancreatic cancer patients.
In this sense, and based on the importance of the development of new products rich in these phytochemicals, Alvarez-Jubete et al. (2014) developed an innovative soup made of broccoli florets or stalks, which reported values of 2147 mg glucoraphanin per kg of broccoli floret soup and 1309 mg glucoraphanin per kg of broccoli stalk soup, as well as 35.5 mg sulforaphane per kg of broccoli floret soup and 12.4 mg sulforaphane per kg of broccoli stalk soup. Such values are quite similar to the ones obtained in the present study, although the beverages developed are not made entirely of broccoli or its by-products, for which it has obtained higher scores in the sensory acceptability (Fig. 3) regarding such authors who recommended further research to optimize the sensory characteristics of their product.
Conclusions
Radish seeds have demonstrated to be an excellent source of bioactive compounds due to their natural richness in sulforaphene, which could be even stimulated with UV-B light as an abiotic stress. In fact, this treatment improved the total glucosinolate and sulforaphene content by ~ 4 and ~ 30% compared to CTRL radish seeds. After their inclusion into a new product, this work demonstrated a great improvement of the nutraceutical content of an innovative tomato cold soup supplemented with UV-B–treated radish seeds, without affecting other quality parameters as colour, pH, TSS, TA, or sensory perception, in which no significant differences were found. Similarly, the microbial load of such products was maintained through the self-life study while a reduction of 0.5 CFU enterobacteria g−1 was observed in formulas with the highest concentrations.
In this sense, the daily consumption of tomato cold soup enriched in 5 g of radish seeds kg−1 is recommended in a balanced diet as it can prevent and/or help in the treatment of oxidative stress and other chronic diseases due to its 12 mg kg−1 fw sulforaphene content and its 33% in vitro bioaccessibility, which has previously been shown to have a positive effect against these diseases.
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
AESAN. (2019). ENIDE: Encuesta Nacional de Ingesta Dietética (2017–2018). AESAN.
Alvarez-Jubete, L., Valverde, J., Kehoe, K., Reilly, K., Rai, D. K., & Barry-Ryan, C. (2014). Development of a novel functional soup rich in bioactive sulforaphane using broccoli (Brassica oleracea L. ssp. italica) florets and byproducts. Food and Bioprocess Technology, 7(5), 1310–1321. https://doi.org/10.1007/s11947-013-1113-9
Artés-Hernández, F., Castillejo, N., & Martínez-Zamora, L. (2022). UV and visible spectrum LED lighting as abiotic elicitors of bioactive compounds in sprouts, microgreens, and baby leaves—a comprehensive review including their mode of action. Foods, 11(3), 1–23. https://doi.org/10.3390/foods11030265
Azarashkan, Z., Motamedzadegan, A., Ghorbani-HasanSaraei, A., Rahaiee, S., & Biparva, P. (2022). Improvement of the stability and release of sulforaphane-enriched broccoli sprout extract nanoliposomes by co-encapsulation into basil seed gum. Food and Bioprocess Technology, 15(7), 1573–1587. https://doi.org/10.1007/s11947-022-02826-z
Campra, P., Aznar-Garcia, M. J., Ramos-Bueno, R. P., Gonzalez-Fernandez, M. J., Khaldi, H., & Garrido-Cardenas, J. A. (2019). A whole-food approach to the in vitro assessment of the antitumor activity of gazpacho. Food Research International, 121, 441–452. https://doi.org/10.1016/j.foodres.2018.11.058
Castillejo, N., Martínez-Hernández, G. B., & Artés-Hernández, F. (2021). Revalorized broccoli by-products and mustard improved quality during shelf life of a kale pesto sauce. Food Science and Technology International, 27(8), 734–745. https://doi.org/10.1177/1082013220983100
Castillejo, N., Martínez-Hernández, G. B., Gómez, P. A., Artés, F., & Artés-Hernández, F. (2016). Red fresh vegetables smoothies with extended shelf life as an innovative source of health-promoting compounds. Journal of Food Science and Technology, 53(3), 1475–1486. https://doi.org/10.1007/s13197-015-2143-2
Cheng, L., Wan, K., Liang, H., & Yuan, Q. (2020). Chapter 9 - Sulforaphane and sulforaphene: Two potential anticancer compounds from glucosinolates. In: Galanakis, C. M., Glucosinolates: Properties, recovery, and applications, Academic Press, 281–312. https://doi.org/10.1016/B978-0-12-816493-8.00009-3
Clarke, J. D., Dashwood, R. H., & Ho, E. (2008). Multi-targeted prevention of cancer by sulforaphane. Cancer Letters, 269(2), 291–304. https://doi.org/10.1016/j.canlet.2008.04.018
Egner, P. A., Chen, J. G., Zarth, A. T., Ng, D. K., Wang, J. B., Kensler, K. H., Jacobson, L. P., Muñoz, A., Johnson, J. L., Groopman, J. D., Fahey, J. W., Talalay, P., Zhu, J., Chen, T. Y., Qian, G. S., Carmella, S. G., Hecht, S. S., & Kensler, T. W. (2014). Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prevention Research, 7(8), 813–823. https://doi.org/10.1158/1940-6207.CAPR-14-0103
Elez-Martínez, P., & Martín-Belloso, O. (2007). Effects of high intensity pulsed electric field processing conditions on vitamin C and antioxidant capacity of orange juice and gazpacho, a cold vegetable soup. Food Chemistry, 102(1), 201–209. https://doi.org/10.1016/j.foodchem.2006.04.048
FAO. (2022). FAOSTAT statistics database. FAOSTAT. Retrieved November 10, 2022 from http://www.fao.org/faostat/en/#data/QC/visualize
Formica-Oliveira, A. C., Martínez-Hernández, G. B., Aguayo, E., Gómez, P. A., Artés, F., & Artés-Hernández, F. (2017). A functional smoothie from carrots with induced enhanced phenolic content. Food and Bioprocess Technology, 10(3), 491–502. https://doi.org/10.1007/s11947-016-1829-4
Formica-Oliveira, A. C., Martínez-Hernández, G. B., Díaz-López, V., Artés, F., & Artés-Hernández, F. (2017). Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biology and Technology, 127, 99–104. https://doi.org/10.1016/j.postharvbio.2016.12.010
Gao, L., Du, F., Wang, J., Zhao, Y., Liu, J., Cai, D., Zhang, X., Wang, Y., & Zhang, S. (2021). Examination of the differences between sulforaphane and sulforaphene in colon cancer: A study based on next-generation sequencing. Oncology Letters, 22(4), 690. https://doi.org/10.3892/ol.2021.12951
González, C. M., Martínez, L., Ros, G., & Nieto, G. (2019). Evaluation of nutritional profile and total antioxidant capacity of the Mediterranean diet of southern Spain. Food Science and Nutrition, 7(12), 3853–3862. https://doi.org/10.1002/fsn3.1211
ISO-8586:2012. (2012). Sensory analysis — general guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors. International Organization for Standardization.
Jacobo-Velázquez, D. A., & Cisneros-Zevallos, L. (2012). An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture (Switzerland), 2(3), 259–271. https://doi.org/10.3390/agriculture2030259
Jansen, M. A. K., & Bornman, J. F. (2012). UV-B radiation: From generic stressor to specific regulator. Physiologia Plantarum, 145(4), 501–504. https://doi.org/10.1111/j.1399-3054.2012.01656.x
Jenkins, G. I. (2009). Signal transduction in responses to UV-B radiation. Annual Review of Plant Biology, 60, 407–431. https://doi.org/10.1146/annurev.arplant.59.032607.092953
Kim, K. H., Moon, E., Kim, S. Y., Choi, S. U., Lee, J. H., & Lee, K. R. (2014). 4-Methylthio-butanyl derivatives from the seeds of Raphanus sativus and their biological evaluation on anti-inflammatory and antitumor activities. Journal of Ethnopharmacology, 151(1), 503–508. https://doi.org/10.1016/j.jep.2013.11.003
Lafarga, T., Ruiz-Aguirre, I., Abadias, M., Viñas, I., Bobo, G., & Aguiló-Aguayo, I. (2019). Effect of thermosonication on the bioaccessibility of antioxidant compounds and the microbiological, physicochemical, and nutritional quality of an anthocyanin-enriched tomato juice. Food and Bioprocess Technology, 12(1), 147–157. https://doi.org/10.1007/s11947-018-2191-5
Lavelli, V., Sri Harsha, P. S. C., Mariotti, M., Marinoni, L., & Cabassi, G. (2015). Tuning physical properties of tomato puree by fortification with grape skin antioxidant dietary fiber. Food and Bioprocess Technology, 8(8), 1668–1679. https://doi.org/10.1007/s11947-015-1510-3
Liang, J., Hänsch, G. M., Hübner, K., & Samstag, Y. (2019). Sulforaphane as anticancer agent: A double-edged sword? Tricky balance between effects on tumor cells and immune cells. Advances in Biological Regulation, 71, 79–87. https://doi.org/10.1016/j.jbior.2018.11.006
Lim, S., Han, S. W., & Kim, J. (2016). Sulforaphene identified from radish (Raphanus sativus L.) seeds possesses antimicrobial properties against multidrug-resistant bacteria and methicillin-resistant Staphylococcus aureus. Journal of Functional Foods, 24, 131–141. https://doi.org/10.1016/j.jff.2016.04.005
Lozanovski, V. J., Houben, P., Hinz, U., Hackert, T., Herr, I., & Schemmer, P. (2014). Pilot study evaluating broccoli sprouts in advanced pancreatic cancer (POUDER trial) - study protocol for a randomized controlled trial. Trials, 15(204), 1–8. https://doi.org/10.1186/1745-6215-15-204
Lozanovski, V. J., Polychronidis, G., Gross, W., Gharabaghi, N., Mehrabi, A., Hackert, T., Schemmer, P., & Herr, I. (2020). Broccoli sprout supplementation in patients with advanced pancreatic cancer is difficult despite positive effects—results from the POUDER pilot study. Investigational New Drugs, 38(3), 776–784. https://doi.org/10.1007/s10637-019-00826-z
Martínez, L., Ros, G., & Nieto, G. (2018). Fe, Zn and Se bioavailability in chicken meat emulsions enriched with minerals, hydroxytyrosol and extra virgin olive oil as measured by Caco-2 cell model. Nutrients, 10(8), 1–12. https://doi.org/10.3390/nu10080969
Martinez-Valverde, I., Periago, M. J., Provan, G., Chesson, A., & Ros, G. (1999). Phenolic compounds in “gazpacho” as function of their ingredients. In Agri-Food Quality II. The Royal Society of Chemistry. https://doi.org/10.1533/9781845698140.6.316
Martínez-Zamora, L., Castillejo, N., & Artés-Hernández, F. (2021a). Postharvest UV-B and photoperiod with blue + red LEDs as strategies to stimulate carotenogenesis in bell peppers. Applied Sciences (Switzerland), 11(9), 3736. https://doi.org/10.3390/app11093736
Martínez-Zamora, L., Castillejo, N., & Artés-Hernández, F. (2021b). Postharvest UV-B and UV-C radiation enhanced the biosynthesis of glucosinolates and isothiocyanates in Brassicaceae sprouts. Postharvest Biology and Technology, 181, 111650. https://doi.org/10.1016/j.postharvbio.2021.111650
Martínez-Zamora, L., Castillejo, N., Gómez, P. A., Artés, F., & Artés-Hernández, F. (2022). An abiotic UV-B stress on Brassicaceae seeds increased their phytochemical content on 7-days sprouts. Journal of Horticulture and Postharvest Research, 2022(4), 297–308. https://doi.org/10.22077/jhpr.2022.5406.1278
Medina-Remón, A., Vallverdú-Queralt, A., Arranz, S., Ros, E., Martínez-González, M. A., Sacanella, E., Covas, M. I., Corella, D., Salas-Salvadó, J., Gómez-Gracia, E., Ruiz-Gutiérrez, V., Lapetra, J., García-Valdueza, M., Arós, F., Saez, G. T., Serra-Majem, L., Pinto, X., Vinyoles, E., Estruch, R., & Lamuela-Raventos, R. M. (2013). Gazpacho consumption is associated with lower blood pressure and reduced hypertension in a high cardiovascular risk cohort. Cross-sectional study of the PREDIMED trial. Nutrition, Metabolism and Cardiovascular Diseases, 23(10), 944–952. https://doi.org/10.1016/j.numecd.2012.07.008
Opara, U. L., Al-Ani, M. R., & Al-Rahbi, N. M. (2012). Effect of fruit ripening stage on physico-chemical properties, nutritional composition and antioxidant components of tomato (Lycopersicum esculentum) cultivars. Food and Bioprocess Technology, 5(8), 3236–3243. https://doi.org/10.1007/s11947-011-0693-5
Parchem, K., Piekarska, A., & Bartoszek, A. (2020). Enzymatic activities behind degradation of glucosinolates. Glucosinolates: Properties, Recovery, and Applications, 79–106. https://doi.org/10.1016/B978-0-12-816493-8.00003-2
Pinilla, M. J., Plaza, L., Sánchez-Moreno, C., De Ancos, B., & Cano, M. P. (2005). Hydrophilic and lipophilic antioxidant capacities of commercial Mediterranean vegetable soups (Gazpachos). Journal of Food Science, 70(1), S60–S65. https://doi.org/10.1111/j.1365-2621.2005.tb09066.x
Planes-Muñoz, D., Frontela-Saseta, C., Ros-Berruezo, G., & López-Nicolás, R. (2021). Effect of gazpacho, hummus and ajoblanco on satiety and appetite in adult humans: A randomised crossover study. Foods. https://doi.org/10.3390/foods10030606
Raiola, A., Errico, A., Petruk, G., Monti, D. M., Barone, A., & Rigano, M. M. (2018). Bioactive compounds in Brassicaceae vegetables with a role in the prevention of chronic diseases. Molecules, 23(1), 15. https://doi.org/10.3390/molecules23010015
Riedl, M. A., Saxon, A., & Diaz-Sanchez, D. (2009). Oral sulforaphane increases phase II antioxidant enzymes in the human upper airway. Clinical Immunology, 130(3), 244–251. https://doi.org/10.1016/j.clim.2008.10.007
Schreiner, M., Martínez-Abaigar, J., Glaab, J., & Jansen, M. (2014). UV-B induced secondary plant metabolites. Optik & Photonik, 9(2), 34–37. https://doi.org/10.1002/opph.201400048
Soundararajan, P., & Kim, J. S. (2018). Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules, 23(11), 2983. https://doi.org/10.3390/molecules23112983
Ströhla, L. C., Hidangmayum, K. S., Waehrens, S. S., Orlien, V., & Petersen, M. A. (2022). Effect of processing and accelerated storage on the volatile composition and sensory profile of a tomato soup. Food Quality and Safety, 6, 1–12. https://doi.org/10.1093/fqsafe/fyac024
Verde Méndez, C. M., Rodríguez Rodríguez, E. M., Díaz Romero, C., Sánchez Mata, M. C., Matallana González, M. C., & Torija Isasa, M. E. (2011). Vitamin C and organic acid contents in Spanish “gazpacho” soup related to the vegetables used for its elaboration process. CYTA - Journal of Food, 9(1), 71–76. https://doi.org/10.1080/19476331003654393
Wang, J., Qiu, Y., Wang, X., Yue, Z., Yang, X., Chen, X., Zhang, X., Shen, D., Wang, H., Song, J., He, H., & Li, X. (2017). Insights into the species-specific metabolic engineering of glucosinolates in radish (Raphanus sativus L.) based on comparative genomic analysis. Scientific Reports, 7(1), 1–9. https://doi.org/10.1038/s41598-017-16306-4
Wang, Y., Wang, Q., Sun, H., Zhang, Z., Qian, H., Zhao, X., He, H., & Zhang, L. (2022). Glucosinolate profiles in different organs of 111 radish accessions and candidate genes involved in converting glucobrassicin to 4-hydroxyglucobrassicin. Journal of Agricultural and Food Chemistry, 70(2), 488–497. https://doi.org/10.1021/acs.jafc.1c05107
Yi, G., Lim, S., Chae, W. B., Park, J. E., Park, H. R., Lee, E. J., & Huh, J. H. (2016). Root glucosinolate profiles for screening of radish (Raphanus sativus L.) genetic resources. Journal of Agricultural and Food Chemistry, 64(1), 61–70. https://doi.org/10.1021/acs.jafc.5b04575
Zhu, X., Healy, L., Zhang, Z., Maguire, J., Sun, D. W., & Tiwari, B. K. (2021). Novel postharvest processing strategies for value-added applications of marine algae. Journal of the Science of Food and Agriculture, 111(11), 4444–4455. https://doi.org/10.1002/jsfa.11166
Acknowledgements
Noelia Castillejo contract was funded by a predoctoral grant (FPU16/04763) from the Spanish Ministry of Education until March 2022. Lorena Martínez-Zamora contract has been financed by the Programme for the Re-qualification of the Spanish University System funded by the EU NextGeneration during the three-year period 2021–2023, Margarita Salas modality by the University of Murcia. The authors thank G’s España Group (Torre-Pacheco, Murcia, Spain) for the kind supply of the Kumato® tomatoes used for these experiments.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Project PID2021-123857OB-I00 financed by the Spanish Ministry of Science and Innovation, the Spanish State Research Agency /10.13039/501100011033/ and FEDER. This work is also a result of the AGROALNEXT program and was supported by MCIN with funding from EU NextGeneration (PRTR-C17.I1) and by Seneca Foundation with funding from Autonomous Community of the Region of Murcia.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: Francisco Artés–Hernández and Lorena Martínez-Zamora performed the experiments: Noelia Castillejo and Lorena Martínez-Zamora; investigation: Francisco Artés–Hernández, Lorena Martínez-Zamora and Noelia Castillejo; software: Lorena Martínez-Zamora; validation: Francisco Artés–Hernández, Lorena Martínez-Zamora and Noelia Castillejo; resources: Francisco Artés–Hernández; data curation: Noelia Castillejo; writing—original draft preparation + review and editing: Francisco Artés–Hernández, Lorena Martínez-Zamora and Noelia Castillejo; visualization: Francisco Artés–Hernández; supervision: Francisco Artés–Hernández; project administration: Francisco Artés–Hernández; funding acquisition: Francisco Artés–Hernández.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Zamora, L., Castillejo, N. & Artés-Hernández, F. Fortification of an Innovative Tomato Cold Soup with High Bioaccessible Sulforaphene from UV-B–Treated Radish Seeds. Food Bioprocess Technol (2023). https://doi.org/10.1007/s11947-023-03273-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-023-03273-0