Abstract
Marination is commonly used to preserve fish, which, in combination with other non-thermal technologies, such as vacuum impregnation and high hydrostatic pressure, may help to preserve freshness and extend shelf life. In addition, marination may mask changes in physicochemical properties and the sensory attributes of fish resulting from intense pressurization treatments. In this study, we evaluated the effects of vacuum impregnation (50 mbar for 5 min) alone or in combination with a moderate pressurization treatment (250 MPa for 6 min) on the physicochemical properties, microbiological and oxidative stability, and sensory properties of refrigerated seabream fillets. Compared to conventional marination, vacuum impregnation alone had no effect on the aforementioned properties, except for a higher perception of lemon aroma (0.9 vs. 1.6). However, vacuum impregnation with pressurization reduced the total viable mesophilic aerobic bacteria to counts below 4 log colony forming units (CFU)/g after 16 days of storage at ≤ 2 °C, compared to 6 log CFU/g with conventional marination. Additionally, the color and texture were affected by the pressurization treatment. However, color was more susceptible, and at the beginning of storage, lightness was higher in the pressurized samples than in the control (52 vs. 78). Regardless, this whitening effect and other minor changes in texture and sensory properties compared to conventional marination with vacuum impregnation with pressurization can be considered of little relevance considering the increase in shelf life, the lack of lipid oxidation (maintained at low and similar levels as those of the non-pressurized samples), and the intrinsic whitening effects of certain marinades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consumer demand for fresh and minimally processed foods, including fishery products, has increased in recent years. Furthermore, considerable research has been conducted regarding the use of various non-thermal technologies and innovative processes to extend the shelf life of products, while ensuring their safety and minimizing changes in sensory characteristics (Zhao et al., 2019b).
Marinating is a traditional food practice that remains common in fish processing, and represents an interesting strategy for the development of minimally processed fresh fish. Furthermore, vacuum impregnation facilitates the penetration of dissolved or suspended substances directly into the porous structure of a product in a controlled manner, allowing for fast compositional and structural changes (Bugueño et al., 2003; Gras et al., 2002). Vacuum impregnation is useful for brining and developing fish new products (Bugueño et al., 2003; Tomac et al., 2020). In addition, vacuum impregnation can incorporate different preservatives into fish fillets (Andrés-Bello et al., 2015; Zhao et al., 2019a). Therefore, the combination of vacuum impregnation and marinating can enhance product preservation by increasing the effectiveness of the marinade by allowing faster penetration into fillets, shortening the preparation process. Moreover, the sensory properties of the initial product can be preserved, since high temperatures are not required.
High hydrostatic pressure (HHP) delays the onset of meat and fish spoilage by inhibiting microbial growth. Therefore, it is widely applied to preserve the meat of several fish species, particularly at pressures above 350 MPa (Garrido et al., 2016; Gómez-Estaca et al., 2018; Yu et al., 2020). At pressures below 350 MPa, it is possible to inactivate trimethylamine-N-oxide demethylase (Gou et al., 2010) and limit H2S-producing and other spoilage bacteria (de Alba et al., 2019; Malinowska-Pańczyk & Kołodziejska, 2016). Although moderate pressures (100–350 MPa) are less effective with regard to microbial inactivation, they may preserve the structural properties of raw fish (Aubourg et al., 2013a, b). An important drawback of HHP is that the surface of the products becomes whiter in color at higher pressure levels and longer exposure times, resulting in a product that looks cooked (Yu et al., 2020). These negative effects can be masked by the cooking effect of the marinade.
Although the application of relatively moderate pressurization treatments in fish and fish products is scarce, the combination with other processing strategies aimed at improving shelf life is of technological interest. Therefore, the objective of this study was to investigate the effects of marination using vacuum impregnation alone or in combination with HHP exposure at 250 MPa for 6 min on the physicochemical properties, microbiological and oxidative stability, and sensory properties of vacuum-packed seabream fillets, a widely consumed fish in the Mediterranean area, during refrigerated storage.
Material and Methods
Raw Materials and Equipment
The seabream (Sparus aurata) used in this study was sea farmed in cages at different farm locations (Grupo Culmarex, Spain). Each fish weighed approximately 400–500 g. After being caught, seabreams were immersed in an ice bath and transported to the processing plant, where they were classified, mechanically descaled, gutted, and filleted. A total of 250 fillets with skin (110 g on average) from 125 animals were kept on ice until the following day. White vinegar was supplied by Borges (Tàrrega, Spain). Lemon concentrate was supplied by Suntory (Pulco, Madrid, Spain). The lemon peel aroma was supplied by Sosa Ingredients (Moià, Spain).
HHP equipment was used to pressurize the samples, using water as a pressure-transmitting medium (Hyperbaric Wave 6500/120; N.C. Hyperbaric, S.A., Burgos, Spain). A prototype machine consisting of a vacuum chamber connected to a vacuum pump was used for the vacuum impregnation treatments.
Marination Solutions and Experimental Design
A marinating solution consisting of 85.5% water, 14% lemon concentrate, 0.6% white vinegar, and 0.08% lemon peel aroma was homogenized with 0.03% ground fish using a kitchen-aided immersion blender. Fillets were randomly assigned to the following treatments: (1) marination control treatment consisting of marination with the lemon–vinegar marinade for 10 min at atmospheric pressure at 4 °C, drained for 5 min, and then vacuum packed (MC); (2) marination treatment consisting of marination with a lemon–vinegar marinade for 5 min at 50 mbar and 4 °C, followed by 5 min at atmospheric pressure, drained for 5 min, and then vacuum packed (vacuum impregnated with lemon and vinegar; VIM); and (3) marination treatment consisting of marination with lemon–vinegar marinade for 5 min at 50 mbar and 4 °C, followed by 5 min at atmospheric pressure, drained for 5 min, vacuum packed, and subsequently exposed to 250 MPa for 6 min at 10 °C (VIM + HHP). A preliminary study was carried out to determine the minimum pressurization treatment that, when compared with unpressurized samples, resulted in microbial counts reduction after few days of refrigerated storage. The mass ratio of fillet sample to marinating solution was maintained at 1:3 (w/v; fish:marinade). After treatment, the samples were stored at 1–2 °C for 1, 5, 9, 12, and 16 days. During each sampling period, four fillets from the same treatment (pooled sample) were randomly selected for microbiological and chemical analyses. The experiment was performed in triplicate.
Microbiological Analysis
Ten grams of fish muscle from four fillets were homogenized (dilution 1:10) with saline peptone water (8.5 g/L NaCl plus 1 g/L Bacto Peptone) in a Stomacher blender bag. The homogenate was serially diluted with saline peptone water. Total viable mesophilic aerobic bacteria (TVC) were incubated at 30 ± 1 °C for 72 h on PCA agar, according to ISO protocol 4833–1:2013. Lactic acid bacteria (LAB) were incubated under anaerobic conditions at 30 ± 1 °C for 72 h on MRS agar according to the ISO protocol 15214:1998. For hydrogen sulfide-producing bacteria (SPB), 1.0 mL of homogenate was poured into 10 mL of iron agar. After setting, a 10 mL overlay of molten media was added. Finally, hydrogen sulfide-producing bacteria (mainly Shewanella putrefaciens) were enumerated on Lyngby Iron Agar after 4 days at 20 °C.
Instrumental Color Measurement
A colorimeter (Chroma Meter CM-600d; Minolta, Tokyo, Japan) was used to measure the color in the CIE-Lab space: lightness (L*), redness (a*), and yellowness (b*). The illuminant used was a D65 with a 10° angle as the standard observer. The color was measured in the dorsal zone of each fillet six times. The mean values of the measurements for each test per animal were retained for statistical analysis.
Instrumental Texture Measurement
A non-destructive compression test (compression rate 30%) with a spherical probe (18.4 mm diameter) was conducted on the top loin of each fillet. The tests were performed using a TA-HD plus Texture Analyzer (Stable Micro System, Surrey, England) at a constant speed of 1 mm/s. The TPA test (spherical probe) was performed on raw samples in three different parts along the fillet. The mean values of the measurements for each test per animal were retained for statistical analysis.
Drip Loss, Water Holding Capacity, and pH
Drip loss was determined by weighing the amount of liquid remaining in each vacuum bag after removing fish fillets. Drip loss was calculated by considering the initial sample weight and vacuum bag weight. Water holding capacity (WHC) was measured in duplicate for each fillet. Each fillet was diced into small pieces [n = 2–3 pieces (approx. 2 g; Ws)], wrapped in two filter papers (also weighted, Wi), and centrifuged (3000 × g, 10 min, 20 °C). After centrifugation, the sample was removed, and the filter papers were weighed (Wf). The WHC was expressed as grams of water in the sample after centrifugation per 100 g of water initially present in the sample:
where H is the moisture content (%). Fillets from the same treatment group (n = 4) were ground together using a kitchen-aid food processor. Moisture was determined by drying at 103 ± 2 °C until a constant weight was reached. The pH of the samples was measured in triplicate using an S40 SevenMulti (Mettler-Toledo SAE, Barcelona, Spain) and an Inlab Solids Pro (Mettler-Toledo SAE) probe. Samples were then vacuum-packed in aluminum bags and stored at − 75 °C for the chemical analysis.
Lipid Hydroperoxides
Ground fish (3 g) were weighed in duplicate in a 50-mL centrifuge tube, to which 5 mL of chloroform and 10 mL of methanol were added. The tube was immediately immersed in ice using Ultra-Turrax T25 (IKA Werke GmbH & Co. KG, Staufen, Germany), and the sample was homogenized at a speed of 3 for 1 min. After adding 5 mL of chloroform, the sample was vortexed for 1 min. Then, 5 mL of water was added, and the mixture was vortexed for 1 min. After centrifugation at 3000 × g for 30 min, the organic phase was filtered through Whatman filter paper (No. 1) in a volumetric flask. Next, 200 μL of this solution was mixed with 2.8 mL of methanol/butanol (2:1, v/v), and the lipid hydroperoxide content was measured, as described previously (Bou et al., 2019). Cumene hydroperoxide was used as a standard, and the results were expressed as mmol of cumene hydroperoxide equivalents kg−1 sample.
Total Volatile Basic Nitrogen (TVBN)
Ground fish (10 g) were weighed for analysis and diluted with 10 mL of distilled water. The mixture was completely homogenized using the Ultra-Turrax T25 model for 30 s at a speed of 3. Next, 10 mL of 150 g/kg trichloroacetic acid and 2 g/L ethylenediaminetetraacetic acid were added to the homogenate, and the mixture was vortexed for 30 s. The mixture was stored at 4 °C for 1 h. After centrifugation (15 min at 1250 × g), the aqueous phase was filtered through Whatman filter paper into a 20-mL volumetric flask. TVBN was determined in aliquots using a segmented continuous flow injection analyzer (Futura System; Alliance Instruments, Frepillon, France). Volatile bases were released from this extract using 3 M sodium hydroxide and distilled water. The distillate was trapped in a phosphoric acid solution (85% phosphoric acid); thus, the lowered acidity of this solution was measured by the oxidoreduction reaction of potassium iodide (50 g/L) and potassium iodate (2 g/L), yielding iodine. The decrease in yellow–brown color was measured at 410 nm. For the calibration curve, ammonium chloride was used between 0 and 150 mg/L N-NH4Cl dissolved in TCA 75 g/L (the results are expressed as mg nitrogen/kg, TVBN). This determination was performed after preparation and at different refrigerated storage points. Each sample was analyzed in duplicate. The mean value for each replicate was used as a single measurement in all analyses.
TBARS
A 2.5-mL aliquot of the filtered extract obtained for TVBN determination was pipetted into a screw-capped amber tube and 2.5 mL of 20 mmol/L aqueous thiobarbituric acid TBA was added. The reaction mixture was incubated for 30 min at 70 °C in a water bath with agitation. After the tube was tempered for 30 min at room temperature, the absorbance was measured at 532 nm using a spectrophotometer. For the calibration curve, 1,1,3,3-tetraethoxypropane was used, and the results were expressed as micrograms of malondialdehyde equivalents kg−1. This determination was carried out for the same storage period as that used for TVBN. Each sample was analyzed in duplicate, and the mean value was recorded as a single measurement.
Lipase Activity
Crude lipases were extracted from ground samples after 1 day of storage, according to a previously described method (Hernández et al., 1999) with some modifications. In brief, 5 g was homogenized in 25 mL of 50 mM phosphate buffer (pH 7.5) plus 5 mM EGTA, using the Ultra-Turrax T25 homogenizer (4 × 10 s at 24,000 rpm) while immersed in ice. The homogenate was stirred for 30 min on ice and then centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant was filtered and diluted to 25 mL with extraction buffer for enzyme activity assays. Neutral and acid lipase activities were measured as previously described (Jin et al., 2010; Zhou et al., 2019). 4-Methylumbellferyl oleate was used as a substrate to measure lipase activity. The substrate liberates fluorescent 4-methylumbelliferone after lipase hydrolysis, which was measured at 350 nm and 445 nm for excitation and emission, respectively, using a Varioskan microplate reader (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Different reaction buffers were used to determine different lipase activities. In the case of neutral lipase, the reaction buffer comprised 0.22 M Tris/HCl (pH 7.5, containing 0.05% (w/v) Triton X-100), whereas for acid lipase, this was 0.1 M disodium phosphate/0.05 M citric acid (pH 5.0, containing 0.05% (w/v) Triton X-100 and 0.8 mg/mL BSA). One unit (U) of activity was defined as 1 nmol of released 4-methylumbelliferone per min at 37 °C.
Sensory Analysis
The sensory evaluation of the samples was carried out by eight selected and trained panelists (ISO 8586–1:1993 and ISO 8586–2:1994). The descriptors for the samples were generated by open discussion in previous sessions, as described in Lazo et al. (2017). The samples were cooked in a convection oven at 115 °C for 20 min (100% relative humidity) in individual transparent glass jars. Samples were coded with three-digit random numbers and presented to the assessors in two different sessions, balancing the first-order and carry-over effects (Macfie et al., 1989). Each panelist assessed three treatments from the same section (front, central, and rear) of different animals in each tasting session. A non-structured scoring scale was used for the analysis, where 0 indicates the absence of the descriptor and 10 indicates the higher intensity of each descriptor.
Statistical Analysis
Two-way analysis of variance (ANOVA) considering the different fish fillet treatments and the storage time (main effects), and their interactions was performed to examine the existence of significant differences in the physicochemical parameters (drip loss, WHC, pH, color, and texture), chemical alteration parameters (lipid hydroperoxides, TBARS, and TVBN), and microbial counts (TVC, LAB, and SPB). The same model, including panelists, sample sections, and sessions as fixed factors, was applied for the sensory attributes. In addition, a series of one-way ANOVA tests was performed for each storage time to determine the existence of significant differences between the different fish treatments. Tukey’s honestly significant difference test was used to identify statistically significant differences within each main effect, considering p < 0.05 as significant.
Results and Discussion
Water Retention and Shelf Life Assessment
Table 1 shows the results in vacuum-packed seabream fish fillets as affected by different treatments and refrigerated storage. Initially, drip loss was lower in the VIM treatment and higher in the VIM + HHP treatment, whereas the MC was not different from the other two treatments. During storage, there was a tendency toward increased drip loss. However, only the MC and VIM treatments were found to be different when comparing their initial and final values. These results are in line with those reported by Hurtado et al. (2000), who found decreased drip loss during the refrigerated storage of pressurized hake at 400 MPa. It is worth mentioning that no differences were observed in moisture content as a consequence of marination treatments and storage time (70.2%, 70.8%, and 69.2% for MC, VIM, and VIM + HHP, respectively). However, these values were lower than those reported for fresh fillets (Alasalvar et al., 2001). This can be attributed to the effect of marination, which leads to reduced water content. Marination can also explain why the WHC was slightly lower than that in other studies dealing with seabream (Campus et al., 2010; Garrido et al., 2016). The fact that all treatments were marinated may have resulted in a similar WHC at the beginning of the storage period (Table 1). However, the VIM + HHP treatment resulted in lower WHC than the other treatments after five or more days of storage. Similarly, as reported by Aubourg et al. (2013a, b), the expressible water content of Atlantic mackerel has been found to increase with HHP treatment. In seabream, Campus et al. (2010) found that the WHC decreased with an increasing pressure (200, 300, and 400 MPa). Ramirez-Suarez and Morrissey (2006) reported that pressurized tuna resulted in a more “loose” gel structure, which facilitated the release of liquids. These effects have been attributed to denaturation changes in myofibril structure, with actin being mainly responsible for these changes. Campus et al. (2010) reported that high-pressure treatments inactivate degrading enzymes acting on proteins related to tissue integrity preservation and contribute to the maintenance of WHC. However, storage time was found to have no effect on WHC when studying the different treatments. Although the effects of sensory and textural properties will be discussed further, pressurization at 250 MPa for 6 min seems to have minimal effects on WHC and drip loss.
At the beginning of storage, MC fillets recorded lower pH values than VIM + HHP fillets (Table 1). The initial pH values of the marinated fillets at the beginning of storage (6.13‒6.25) were similar to those reported in previous studies using fresh seabream fillets (Garrido et al., 2016; Giannoglou et al., 2021). Giannoglou et al. (2021) also reported a slight increase (from 6.33 to 6.44) as a result of pressurization treatment (300 MPa for 5 min). The pH of the marinade was 2.6, suggesting that both the relatively short time of marination and vacuum impregnation resulted in the relatively poor diffusion of the marinade inside the seabream fillets. This is also supported by the lack of an effect at longer storage periods. In contrast, the pH was found to be steady in all treatments for up to 12 days of storage and then increased at the end of storage. The formation of trimethylamine and other basic volatiles by the action and metabolism of endogenous or microbial enzymes explains the increase in pH during storage (Olatunde & Benjakul, 2018). However, TVBN was unaffected by different treatments and storage times (Table 1). The initial TVBN values were similar to those reported in the literature for seabream and other species (Erkan & Üretener, 2010; Garrido et al., 2016; Parlapani et al., 2015). Moreover, the amounts reached at the end of storage were below the limit of 30 mg N/100 g (European Commission, 2005). In fact, this parameter typically increases during the late stages of storage, making it suitable only as an acceptance/rejection criterion. It is well known that the addition of individual or mixtures of different organic acids, such as citric, ascorbic, and acetic, can serve as effective antimicrobials in fish (García-Soto et al., 2014; Mei et al., 2019). These findings suggest that minor degradation occurred in marinated fillets, which can be partly attributed to the protective effect of the lemon–vinegar marinade.
The effects of different treatments and storage times on microbial counts are shown in Fig. 1. A limit of 6 log CFU/g was reached for the MC and VIM treatments at the end of storage. Therefore, it appears that under the studied conditions, vacuum impregnation does not offer a technological advantage with regard to microbial growth. However, pressurization at 250 MPa caused a decrease in the TVC. This microbial reduction was also observed at different storage times when comparing different treatments. However, TVC was not completely inactivated and increased with storage time. These findings are in line with the reported decrease in TVC in mackerel and seabream fillets upon pressurization at 300 MPa for 5 min (de Alba et al., 2019; Giannoglou et al., 2021). Similarly, exposure to 200 MPa for 2 min was found to reduce aerobic psychrotrophic counts in salmon, cod, and mackerel species (Rode & Hovda, 2016). With regard to LAB, the initial low values were in agreement with those reported in other studies (Garrido et al., 2016; Giannoglou et al., 2021). Therefore, it is unclear whether pressurization at 250 MPa is an effective strategy for reducing LAB counts, as suggested by previous studies in the range of 200–300 MPa and for 5–10 min (Amanatidou et al., 2000; Giannoglou et al., 2021). Conversely, previous studies also reported the resistance of this microbial group to HHP treatment (250 MPa for 15 min) (Gómez-Estaca et al., 2018). Regardless of the treatment, the LAB counts remained unchanged during storage (Fig. 1). These results are in line with those of Giannoglou et al. (2021), who reported a more progressive and delayed increase in LAB counts in pressurized fillets than in TVC. The initial SPB counts were lower than those reported in other studies on seabream and other fish species (Carrascosa et al., 2015; Gómez-Estaca et al., 2018; Parlapani et al., 2015). Similar to LAB, the inactivation of SPB is difficult to ascertain because of its relatively low levels, which explains why no differences were observed in SPB counts between treatments for up to 5 days of storage. After this storage time, the VIM + HHP treatment recorded lower counts than the other treatments, which demonstrates the effectiveness of high pressures against SPB, in agreement with previous studies at pressures ≤ 250 MPa (Amanatidou et al., 2000; Gómez-Estaca et al., 2018). Similar to other studies, HHP treatment maintained SPB counts below the detection limit throughout storage (Gómez-Estaca et al., 2018). Therefore, the studied pressurization treatment (250 MPa, 6 min) offered good protection in front of different microbial groups and more specifically against SPB, which allowed for the extension of the shelf life of the marinated product for up to 16 days under the experimental conditions. However, vacuum impregnation does not offer an additional advantage in controlling microbial growth compared with conventional marination at atmospheric pressure.
Total aerobic viable counts (a), lactic acid bacteria counts (b), and sulfide-producing bacteria counts (c) of vacuum-packed seabream fillets stored under refrigeration. Different capital letters (A–B) indicate significant differences within a storage time whereas different lowercase letters (a–c) indicate significant differences within a treatment (p ≤ 0.05)
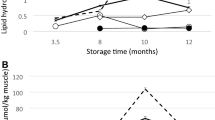
In addition to microbial spoilage, the progression of oxidation may determine the shelf life of fish products. The results of oxidation, as measured by the content of lipid hydroperoxides and TBARS values, are shown in Table 1. The lipid hydroperoxide content was similar among treatments at all storage times. It can also be observed that the lipid hydroperoxide content showed a trend toward higher values with longer storage periods. However, only the fillets from the VIM + HHP treatment were found to be significantly different at the end of the storage period. It has been widely reported that HHP can promote oxidation, which has been attributed to cell membrane damage and the denaturation of heme proteins, leading to hemin release (Bou et al., 2019; Gómez-Estaca et al., 2018; Oliveira et al., 2017). Similar to lipid hydroperoxides, TBARS showed no differences among treatments at all storage times. However, TBARS values increased progressively with storage time, regardless of the treatment, and all recorded higher values than at the beginning of storage. However, the progression of lipid oxidation can be considered low when compared with other studies evaluating fresh and pressurized seabream fillets (Erkan & Üretener, 2010; Giannoglou et al., 2021). For instance, Erkan and Üretener (2010) who compared non-pressurized and pressurized seabream fillets at 250 MPa for 5 min at 3 °C and 15 °C reported higher TBARS values after 19 days of refrigerated storage (1.5–3.5 mg/kg) than in the present study. Giannoglou et al. (2021) found that fish exposed to 300 MPa for 5 min had lower values than unpressurised controls throughout the storage period. Rode and Hovda (2016) compared pressurization at 0, 200, and 500 MPa for different fish species. These authors reported that samples exposed to 500 MPa had higher TBARS values. However, the progression of lipid oxidation depended on the fish species and, in some cases, the pro-oxidant effect at 200 MPa was similar to that of the control. Espinosa et al. (2015) cooked and pressurized seabream fillets at 300 and 600 MPa for 5 min in a sauce containing olive oil and vinegar, among other ingredients, and compared the results. The TBARS values in this previous study are in line with our findings and increased with storage time; however, in most cases, the authors did not find significant differences between treatments. Among other factors, the partial inactivation of lipases (5.4 ± 0.11, 6.1 ± 0.59, and 2.9 ± 0.87 U kg−1 for neutral lipase in MC, VIM, and VIM + HHP, respectively, and 2.2 ± 0.16, 2.3 ± 0.46, and 1.5 ± 0.46 U kg−1 sample for acid lipase in MC, VIM, and VIM + HHP, respectively) as a consequence of pressurization treatment may have reduced the release of free fatty acids, which are known to be prone to oxidation (Vázquez et al., 2018; Zhou et al., 2019). In addition, it is worth mentioning that the marinade used in the current study contains lemon, since citric acid and ascorbic acid have well-known antioxidant properties (Mei et al., 2019). Therefore, it is reasonable to assume that in seabream fillets, marination together with exposure to relatively mild pressurization treatments provided sufficient protection against the progression of lipid oxidation, with microbial growth being the main factor determining shelf life.
Instrumental Color, Instrumental Texture, and Sensory Analysis
The instrumental colors of the fish fillets are shown in Table 2. The lightness, ranging from 52 to 78, was higher than that in previous studies using seabream fillets, which ranged from 35 to 45 (Andrés-Bello et al., 2015; Giannoglou et al., 2021). This increase can be attributed to the marinade cooking effect as shown in Fig. 2. Moreover, the comparison between treatments showed that pressurization (250 MPa, 6 min) increased the lightness, an effect that was maintained throughout the storage period. This finding agrees with other studies that have attributed this whitening effect to protein changes, leading to an increase in light reflection (Giannoglou et al., 2021; Oliveira et al., 2017). However, mackerel pressurized at 150 MPa for 2.5 min or coho salmon pressurized at 200 MPa for 30 s had no effect on lightness after processing (Aubourg et al., 2013a, b). Lightness was unaffected during the storage of non-pressurized samples (MC, VIM), whereas VIM + HHP samples showed a tendency toward darker colors. It is unclear whether this effect was caused by the browning of heme pigments and the progression of lipid oxidation and/or a lower water content with longer storage times. With regard to redness, the recorded values at the beginning of storage were lower than those reported for fresh seabream fillets (Andrés-Bello et al., 2015; Oliveira et al., 2017). In addition, VIM + HHP treatment resulted in fillets that were more reddish than VIM, whereas MC showed intermediate values. Conversely, various studies have reported that HHP causes a decrease in redness, particularly at an elevated pressure (Erkan & Üretener, 2010; Giannoglou et al., 2021; Zhao et al., 2019b). Therefore, it is possible that under these experimental conditions, a marinade may be more effective than HHP in decreasing redness. It is also worth mentioning that fish contain relatively high amounts of hemoglobin, which has been shown to be relatively stable at pressures below 300 MPa (Bou et al., 2019). Upon storage, redness increased in all treatments, and at the end of the storage period, no differences were observed between treatments. Some unclear behaviors in redness values have also been reported as a result of pressurization and vacuum impregnation treatments (Andrés-Bello et al., 2015; Erkan & Üretener, 2010). As the color red is supposed to be mainly determined by heme proteins, this increase in redness may be attributed to the combination of vacuum and the reducing capacity of certain compounds, such as ascorbic acid, from the marinade. The yellowness values of the MC and VIM samples were similar to those reported for seabream fillets (Andrés-Bello et al., 2015; Erkan & Üretener, 2010). Andrés-Bello et al. (2015) reported that vacuum impregnation with nisin results in a decrease in yellowness. Hence, it is possible that under our experimental conditions, marination may have counteracted this effect. However, pressurization led to an increased yellowness in the product, which was maintained throughout the storage period (Table 2). This effect has been previously described in various fish species (Erkan & Üretener, 2010; Oliveira et al., 2017; Zhao et al., 2019b). In addition, there was a tendency for higher yellowness values with storage time, regardless of the treatment, which is also in agreement with similar studies (Erkan & Üretener, 2010). An increase in this parameter is often related to the progression of lipid oxidation (Aubourg et al., 2013a, b).
The instrumental textures are shown in Table 2, which indicate that hardness was unaffected by the different treatments at almost all storage times. Only after 5 days of storage, the VIM + HHP treatment resulted in a harder texture than the other treatments. These findings are in line with those reported in previous studies, in which the application of low-pressure treatments (200 MPa for 30 s) in coho salmon was evaluated (Aubourg et al., 2013a, b). However, the application of HHP normally results in an increase in hardness (Giannoglou et al., 2021; Oliveira et al., 2017; Zhao et al., 2019a, b). Nonetheless, Oliveira et al. (2017) found that the effects of HHP are dependent on the process parameters, fish species, and methodology used. Therefore, it seems that, in our study, HHP caused minimal changes in proteins. During storage, there is a tendency toward a softer texture, which can be attributed to proteolysis. This effect was not observed in the pressurized samples, suggesting that the proteases were inactivated (Campus et al., 2010). Similar findings were obtained for adhesiveness, with minimal changes observed between the different treatments after 1, 9, and 12 days of storage. However, pressurized treatments caused lower adhesiveness after 5 and 16 days of storage. This parameter also seemed to increase with longer storage times in the MC and VIM samples, whereas the VIM + HHP samples remained unchanged when comparing the values at the beginning and end of the storage period. Cohesiveness was found to behave similarly to adhesiveness, and thus, minimal changes occurred with exposure to the different treatments and their storage. Hence, it can be assumed that different treatments caused minimal changes in these two parameters. Springiness was affected by pressurization for all storage times (Table 2). Therefore, this parameter may better reflect protein changes caused by HHP. In addition, this parameter remained unchanged with storage time in the VIM + HHP samples, whereas lower values were observed in the MC and VIM samples at the end of the storage period. With regard to gumminess, no changes were observed in the different treatments. In addition, the pressurized samples (VIM + HHP) remained unchanged with storage, whereas the MC and VIM samples were found to decrease. The fact that springiness and gumminess remained unchanged in pressurized samples, while there was a trend toward lower values in the MC and VIM samples, may be attributed to the progression of proteolysis and its inactivation by HHP (Campus et al., 2010). Therefore, exposure to HHP had minimal or no effects on seabream fillets, except for springiness, but may have the advantage of preserving textural properties during storage by minimizing the effect of proteolysis.
The sensory analyses of cooked seabream fish fillets are shown in Table 3. Apart from odors imbued by the lemon and vinegar, the characteristic aroma of the fish fillets was unaffected by the treatments. As the marinade contains lemon juice and vinegar, this explains the higher scores in the treatments that involved the marination step (MC, VIM, and VIM + HHP). However, the lemon odor in the MC was not different from that in the unprocessed control sample, suggesting that vacuum impregnation was more efficient in the penetration of the aroma into the tissue. The same trend was observed for vinegar aroma. Unprocessed control samples also showed a lower amount of exudate, although this was only significantly different from the VIM + HHP treatment. High pressures induce protein unfolding and denaturation, which may explain the higher release of exudates. In general, WHC is related to the compression of fibers, and protein denaturation and HHP may alter the conformation of proteins (Campus et al., 2010; Oliveira et al., 2017). These effects may have been exacerbated in the cooked samples, which may explain the increased amount of exudate. Pressurized samples also showed a higher presence of fat droplets when compared with the unprocessed control sample and MC, which may be indicative of structural damage. However, the turbidity of the exudate in the VIM + HHP and unprocessed control samples was higher than that in the MC and VIM samples, whereas no differences were observed in the color of the exudate or the presence of white spots. In general, the appearance of the cooked product (e.g., white spots, color intensity, and laminar structure) was unaffected by the treatments (Table 3). Flavor attributes showed similar results, and the overall flavor intensity, sardine flavor, butter flavor, and bitter flavor were similar between the treatments and the unprocessed control sample. Similar to aroma, sour and lemon flavors were affected by the marinade. The unprocessed control samples recorded the lowest scores, followed by MC, VIM, and VIM + HHP. This finding reinforces the hypothesis of the higher penetration of lemon aroma compounds and acetic acid into fish tissues after vacuum impregnation. In addition, pressurization may enhance the perception of these flavor compounds. Thus, this treatment may also reduce the marination time and improve the sensory characteristics. Firmness, crumbliness, and adherence to teeth were unaffected by the treatments, suggesting that mild pressurization treatments (250 MPa, 6 min) did not substantially change the sensory properties of cooked fillets, which is in agreement with previous studies comparing the effects of 0, 300, and 600 MPa on firmness (Espinosa et al., 2015). The same authors reported that juiciness decreased with pressure; however, in this study, VIM + HHP and unprocessed control fillets were found to be higher. The positive effect of HHP may be related to enhanced perception of lemon and sour attributes. However, marination seems to increase pastiness, which can be attributed to the effect of the marinade rather than exposure to high pressure.
Conclusions
Collectively, these results indicate that the exposure of seabream to 250 MPa for 6 min caused minimal changes in its physicochemical properties (e.g., WHC, drip loss, and texture), TVBN, and oxidation. The main limitation of pressurized fresh fillets could be mainly determined by the increased lightness and more “cooked” appearance, which was higher than that in the other marinated treatments. This effect is related to protein changes which, on the other hand, seem to be of minor importance as most of the textural parameters were mainly unaffected when compared with the other marination processes. These findings are in line with those observed in the sensory analysis, as marination and HHP were found to have minor effects on the studied descriptors, with aroma and flavor attributes mainly determined by marination. However, pressurization treatment extended the shelf life up to 16 days when stored at 1–2 °C, whereas vacuum impregnation was found to be ineffective in controlling microbes compared to conventional marination. Therefore, pressurization at moderate pressures seems to be an effective strategy for extending the shelf life of marinated products, as most of these changes can be minimized or masked by the marinade. However, further studies are required to confirm whether these changes will have an effect on consumer acceptance.
Data Availability
The datasets generated during the current study are available from the corresponding author on request.
References
Alasalvar, C., Taylor, K. D. A., Öksüz, A., Garthwaite, T., Alexis, M. N., & Grigorakis, K. (2001). Freshness assessment of cultured sea bream (Sparus aurata) by chemical, physical and sensory methods. Food Chemistry, 72(1), 33–40. https://doi.org/10.1016/S0308-8146(00)00196-5
Amanatidou, A., Schlüter, O., Lemkau, K., Gorris, L. G. M., Smid, E. J., & Knorr, D. (2000). Effect of combined application of high pressure treatment and modified atmospheres on the shelf life of fresh Atlantic almon. Innovative Food Science and Emerging Technologies, 1(2), 87–98. https://doi.org/10.1016/S1466-8564(00)00007-2
Andrés-Bello, A., de Jesús, C., García-Segovia, P., Pagán-Moreno, M. J., & Martínez-Monzó, J. (2015). Vacuum impregnation as a tool to introduce biopreservatives in gilthead sea bream fillets (Sparus aurata). LWT, 60(2), 758–765. https://doi.org/10.1016/J.LWT.2014.09.063
Aubourg, S. P., Rodríguez, A., Sierra, Y., Tabilo-Munizaga, G., & Pérez-Won, M. (2013a). Sensory and physical changes in chilled farmed coho salmon (Oncorhynchus kisutch): Effect of previous optimized hydrostatic high-pressure conditions. Food and Bioprocess Technology, 6(6), 1539–1549. https://doi.org/10.1007/s11947-012-0799-4
Aubourg, S. P., Torres, J. A., Saraiva, J. A., Guerra-Rodríguez, E., & Vázquez, M. (2013b). Effect of high-pressure treatments applied before freezing and frozen storage on the functional and sensory properties of Atlantic mackerel (Scomber scombrus). LWT - Food Science and Technology, 53(1), 100–106. https://doi.org/10.1016/j.lwt.2013.01.028
Bou, R., Llauger, M., Joosse, R., & García-Regueiro, J. A. (2019). Effect of high hydrostatic pressure on the oxidation of washed muscle with added chicken hemoglobin. Food Chemistry, 292, 227–236. https://doi.org/10.1016/j.foodchem.2019.04.067
Bugueño, G., Escriche, I., Martínez-Navarrete, N., del Mar Camacho, M., & Chiralt, A. (2003). Influence of storage conditions on some physical and chemical properties of smoked salmon (Salmo salar) processed by vacuum impregnation techniques. Food Chemistry, 81(1), 85–90. https://doi.org/10.1016/S0308-8146(02)00381-3
Campus, M., Addis, M. F., Cappuccinelli, R., Porcu, M. C., Pretti, L., Tedde, V., Secchi, N., Stara, G., & Roggio, T. (2010). Stress relaxation behaviour and structural changes of muscle tissues from gilthead sea bream (Sparus aurata L.) following high pressure treatment. Journal of Food Engineering, 96(2), 192–198. https://doi.org/10.1016/j.jfoodeng.2009.07.013
Carrascosa, C., Millán, R., Saavedra, P., Jaber, J. R., Raposo, A., Pérez, E., Montenegro, T., & Sanjuán, E. (2015). Microbiological evolution of gilthead sea bream (Sparus aurata) in Canary Islands during ice storage. Journal of Food Science and Technology, 52(3), 1586–1593. https://doi.org/10.1007/s13197-013-1166-9
de Alba, M., Pérez-Andrés, J. M., Harrison, S. M., Brunton, N. P., Burgess, C. M., & Tiwari, B. K. (2019). High pressure processing on microbial inactivation, quality parameters and nutritional quality indices of mackerel fillets. Innovative Food Science and Emerging Technologies, 55, 80–87. https://doi.org/10.1016/j.ifset.2019.05.010
European Commission. (2005). Commission Regulation (EC) No 2074/2005 of 5 December 2005. Official Journal of the European Union, L 338/27.
Erkan, N., & Üretener, G. (2010). The effect of high hydrostatic pressure on the microbiological, chemical and sensory quality of fresh gilthead sea bream (Sparus aurata). European Food Research and Technology, 230(4), 533–542. https://doi.org/10.1007/s00217-009-1193-y
Espinosa, M. C., Díaz, P., Linares, M. B., Teruel, M. R., & Garrido, M. D. (2015). Quality characteristics of sous vide ready to eat seabream processed by high pressure. LWT - Food Science and Technology, 64(2), 657–662. https://doi.org/10.1016/j.lwt.2015.06.027
García-Soto, B., Böhme, K., Barros-Velázquez, J., & Aubourg, S. P. (2014). Inhibition of quality loss in chilled megrim (Lepidorhombus whiffiagonis) by employing citric and lactic acid icing. International Journal of Food Science & Technology, 49(1), 18–26. https://doi.org/10.1111/ijfs.12268
Garrido, M. D., Hernández, M. D., Espinosa, M. C., & López, M. B. (2016). Enhanced quality characteristics of refrigerated seabream (Sparus aurata) fillets packed under different systems (modified atmosphere vs. vacuum). Journal of Aquatic Food Product Technology, 25(2), 156–168. https://doi.org/10.1080/10498850.2013.838814
Giannoglou, M., Dimitrakellis, P., Efthimiadou, Α, Gogolides, Ε, & Katsaros, G. (2021). Comparative study on the effect of cold atmospheric plasma, ozonation, pulsed electromagnetic fields and high-pressure technologies on sea bream fillet quality indices and shelf life. Food Engineering Reviews, 13(1), 175–184. https://doi.org/10.1007/s12393-020-09248-7
Gómez-Estaca, J., López-Caballero, M. E., Martínez-Bartolomé, M. Á., de Lacey, A. M. L., Gómez-Guillen, M. C., & Montero, M. P. (2018). The effect of the combined use of high pressure treatment and antimicrobial edible film on the quality of salmon carpaccio. International Journal of Food Microbiology, 283, 28–36. https://doi.org/10.1016/j.ijfoodmicro.2018.06.015
Gou, J., Lee, H. Y., & Ahn, J. (2010). Effect of high pressure processing on the quality of squid (Todarodes pacificus) during refrigerated storage. Food Chemistry, 119(2), 471–476. https://doi.org/10.1016/j.foodchem.2009.06.042
Gras, M., Vidal-Brotóns, N., Betoret, A., & Chiralt, & Fito, P. (2002). The response of some vegetables to vacuum impregnation. Innovative Food Science and Emerging Technologies, 3(3), 263–269. https://doi.org/10.1016/S1466-8564(02)00032-2
Hernández, P., Navarro, J. L., & Toldra, F. (1999). Lipolytic and oxidative changes in two Spanish pork loin products: Dry-cured loin and pickled-cured loin. Meat Science, 51(2), 123–128. https://doi.org/10.1016/S0309-1740(98)00108-9
Hurtado, J. L., Montero, P., & Borderias, A. J. (2000). Extension of shelf life of chilled hake (Merluccius capensis) by high pressure/Prolongación de la vida útil de merluza (Merluccius capensis) sometida a altas presiones conservada en refrigeración. Food Science and Technology International, 6(3), 243–249. https://doi.org/10.1177/108201320000600307
Jin, G., Zhang, J., Yu, X., Zhang, Y., Lei, Y., & Wang, J. (2010). Lipolysis and lipid oxidation in bacon during curing and drying-ripening. Food Chemistry, 123(2), 465–471. https://doi.org/10.1016/j.foodchem.2010.05.031
Lazo, O., Guerrero, L., Alexi, N., Grigorakis, K., Claret, A., Pérez, J. A., & Bou, R. (2017). Sensory characterization, physico-chemical properties and somatic yields of five emerging fish species. Food Research International, 100, 396–406. https://doi.org/10.1016/j.foodres.2017.07.023
Macfie, H., Bratchell, N., Greenhoff, K., & Vallis, L. V. (1989). Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. Journal of Sensory Studies, 4, 129–148. https://doi.org/10.1111/j.1745-459X.1989.tb00463.x
Malinowska-Pańczyk, E., & Kołodziejska, I. (2016). The effect of high pressure on formation of volatile amines in minced meat of cod (Gadus morhua). European Food Research and Technology, 242(3), 415–420. https://doi.org/10.1007/s00217-015-2552-5
Mei, J., Ma, X., & Xie, J. (2019). Review on natural preservatives for extending fish shelf life. In Foods (Vol. 8, Issue 10). MDPI Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/foods8100490
Olatunde, O. O., & Benjakul, S. (2018). Natural preservatives for extending the shelf-life of seafood: A revisit. In Comprehensive Reviews in Food Science and Food Safety (Vol. 17, Issue 6, pp. 1595–1612). Blackwell Publishing Inc. https://doi.org/10.1111/1541-4337.12390
Oliveira, F. A. de, Neto, O. C., Santos, L. M. R. dos, Ferreira, E. H. R., & Rosenthal, A. (2017). Effect of high pressure on fish meat quality — A review. In Trends in Food Science and Technology (Vol. 66, pp. 1–19). Elsevier Ltd. https://doi.org/10.1016/j.tifs.2017.04.014
Parlapani, F. F., Haroutounian, S. A., Nychas, G. J. E., & Boziaris, I. S. (2015). Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2°C. Food Microbiology, 50, 44–53. https://doi.org/10.1016/j.fm.2015.03.006
Ramirez-Suarez, J. C., & Morrissey, M. T. (2006). High hydrostatic pressure and heat treatment effects on physicochemical characteristics of albacore tuna (Thunnus alalunga) minced muscle. Journal of Aquatic Food Product Technology, 15(1), 5–17. https://doi.org/10.1300/J030v15n01_02
Rode, T. M., & Hovda, M. B. (2016). High pressure processing extend the shelf life of fresh salmon, cod and mackerel. Food Control, 70, 242–248. https://doi.org/10.1016/j.foodcont.2016.05.045
Tomac, A., Mallo, S. R., Perez, S., Loredo, A. B. G., & Yeannes, M. I. (2020). Vacuum impregnation in Merluccius hubbsi hake fillets brining. Effect on mass transfer kinetics, texture and colour. LWT, 119, 108892. https://doi.org/10.1016/j.lwt.2019.108892
Vázquez, M., Fidalgo, L. G., Saraiva, J. A., & Aubourg, S. P. (2018). Preservative effect of a previous high-pressure treatment on the chemical changes related to quality loss in frozen hake (Merluccius merluccius). Food and Bioprocess Technology, 11(2), 293–304. https://doi.org/10.1007/s11947-017-2010-4
Yu, D., Wu, L., Regenstein, J. M., Jiang, Q., Yang, F., Xu, Y., & Xia, W. (2020). Recent advances in quality retention of non-frozen fish and fishery products: A review. In Critical Reviews in Food Science and Nutrition (Vol. 60, Issue 10, pp. 1747–1759). Taylor and Francis Inc. https://doi.org/10.1080/10408398.2019.1596067
Zhao, X., Zhou, Y., Zhao, L., Chen, L., He, Y., & Yang, H. (2019a). Vacuum impregnation of fish gelatin combined with grape seed extract inhibits protein oxidation and degradation of chilled tilapia fillets. Food Chemistry, 294, 316–325. https://doi.org/10.1016/j.foodchem.2019.05.054
Zhao, Y. M., de Alba, M., Sun, D. W., & Tiwari, B. (2019b). Principles and recent applications of novel non-thermal processing technologies for the fish industry—A review. In Critical Reviews in Food Science and Nutrition (Vol. 59, Issue 5, pp. 728–742). Taylor and Francis Inc. https://doi.org/10.1080/10408398.2018.1495613
Zhou, X., Zhou, D. Y., Liu, Z. Y., Yin, F. W., Liu, Z. Q., Li, D. Y., & Shahidi, F. (2019). Hydrolysis and oxidation of lipids in mussel Mytilus edulis during cold storage. Food Chemistry, 272, 109–116. https://doi.org/10.1016/j.foodchem.2018.08.019
Acknowledgements
We thank our laboratory technician Elvira Tenorio, and the students Chrysoula Kokkou, Ana Clara Bortoluzzi Teixeira, and Mariarosa Colucci, who helped in the analyses of this, and other fish species studied in this project.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the European Union’s Horizon2020 NewTechAqua project (grant agreement no. 862658). Partial financial support was received from the CERCA Program from the Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
RB: investigation; formal analysis; conceptualization; supervision; resources; validation; writing—original draft and editing. LG: investigation; manuscript revision; project administration; funding acquisition. ML: conceptualization; manuscript revision; project administration; funding acquisition. AC: investigation; writing—review and editing. LL-M: investigation; formal analysis. MC: conceptualization; manuscript revision; project administration; funding acquisition.
Corresponding author
Ethics declarations
Consent to Participate
Informed consent was obtained from all subjects involved in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bou, R., Guerrero, L., López, M. et al. Effect of Vacuum Impregnation and High Hydrostatic Pressure Treatments on Shelf Life, Physicochemical, and Sensory Properties of Seabream Fillets. Food Bioprocess Technol 16, 1089–1100 (2023). https://doi.org/10.1007/s11947-022-02980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02980-4