Abstract
Purpose of Review
Fibromyalgia syndrome (FMS) is a disease of unknown pathophysiology, with the diagnosis being based on a set of clinical criteria. Proteomic analysis can provide significant biological information for the pathophysiology of the disease but may also reveal biomarkers for diagnosis or therapeutic targets. The present systematic review aims to synthesize the evidence regarding the proteome of adult patients with FMS using data from observational studies.
Recent Findings
An extensive literature search was conducted in MEDLINE/PubMed, CENTRAL, and clinicaltrials.gov from inception until November 2022. The study protocol was published in OSF. Two independent reviewers evaluated the studies and extracted data. The quality of studies was assessed using the modified Newcastle–Ottawa scale adjusted for proteomic research. Ten studies fulfilled the protocol criteria, identifying 3328 proteins, 145 of which were differentially expressed among patients with FMS against controls. The proteins were identified in plasma, serum, cerebrospinal fluid, and saliva samples. The control groups included healthy individuals and patients with pain (inflammatory and non-inflammatory).
Summary
The most important proteins identified involved transferrin, α-, β-, and γ-fibrinogen chains, profilin-1, transaldolase, PGAM1, apolipoprotein-C3, complement C4A and C1QC, immunoglobin parts, and acute phase reactants. Weak correlations were observed between proteins and pain sensation, or quality of life scales, apart from the association of transferrin and a2-macroglobulin with moderate-to-severe pain sensation. The quality of included studies was moderate-to-good. FMS appears to be related to protein dysregulation in the complement and coagulation cascades and the metabolism of iron. Several proteins may be dysregulated due to the excessive oxidative stress response.
Similar content being viewed by others
Introduction
Fibromyalgia syndrome (FMS) is a rheumatic disease (ICD-10 M79.7) of unknown etiology, characterized by chronic widespread pain accompanied by potential neuroinflammation [1], fatigue, stress [2], memory loss, sleep disturbance, and multiple physical symptoms [3]. The prevalence of FMS in the general population ranges between 2 and 3% [4], but higher ratios have been reported in specific population groups [5]. For example, 14.8% of patients with type 2 diabetes mellitus and 80% of those with Behçet’s disease are diagnosed with FMS [4, 5]. There is however, no gold standard for the diagnostic procedure. Individuals are diagnosed based on clinical criteria suggested by the American College of Rheumatology (ACR), conceived in 1990, and revised in 2010, 2011, and 2016 [6,7,8,9]. The ACR 1990 [7] criteria rely on a clinical examination and the existence of tender points, while the 2010 [6] criteria focus on other disease parameters, including fatigue and sleep disturbances. Aside from the diagnosis, no objective biomarkers or tests have been identified to facilitate a more accurate diagnostic process or mediate the development of a precise prognostic model for FMS.
In medicine, biological markers can be used for disease detection and the discovery of drugs, as well as for monitoring the progress of patients [10]. Although numerous studies suggest plausible mechanisms driving disease development, definite evidence has been relatively scarce. FMS seems to be associated with altered central nervous system (CNS) processing, enhanced excitability, and decreased inhibition [11]. Oxidative stress, vitamin dysregulation, inflammation, autonomic dysfunction, and genetic factors may provide an insight into the pathophysiology [12,13,14,15,16,17]. Proteomics, identifying protein markers in biological fluids, can provide critical information in such complex conditions/diseases [10, 18], like FMS [19].
The present systematic review aimed to provide a comprehensive summary of the proteome of adult patients with FMS, in an effort to shed light on the pathophysiology of the condition, identify diagnostic and prognostic protein markers, and establish some potential therapeutic targets.
Materials and Methods
Systematic Review Protocol and PEO
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [20] and the Synthesis Without Meta-analysis (SWiM) extension [21] were used. By November 2022, the study’s protocol was published at the Center for Open Science Framework (OSF) (https://shorturl.at/rHN45). The Population-Exposure-Outcome (PEO) of the research question is detailed in Supplementary Table 1.
Search Strategy and Algorithm
Two independent reviewers (A.G. and S.G.T.) identified studies through PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), clinicaltrials.gov databases, and the grey literature from inception until November 2022. A senior reviewer (M.G.G.) resolved any discrepancies.
To identify studies in databases, we used a combination of keywords using medical subject headings (MeSH) and free text. The search was conducted in English. The keywords and search syntaxes are listed in Supplementary Tables 2 and 3, respectively.
The Rayyan [22] software was used to identify all studies fulfilling inclusion criteria and to remove duplicates. After all identified studies were imported to the software, titles and abstracts were screened to examine whether inclusion criteria were met. The remaining studies were assessed in full text.
Inclusion and Exclusion Criteria
Studies were included in the systematic review when (1) they were observational studies on FMS, (2) of any duration, (3) in adult patients, (4) by assessing the proteome in biological fluids, (5) published until November 2022, (6) and written in the English language.
Studies were excluded when (1) they were published in another language, (2) pooling patients with FMS together with other chronic pain disorders, (3) including pediatric patients, and (4) animal or preclinical studies.
Outcomes of Interest
Any protein identified in body fluids through proteomics in patients with FMS compared to controls was considered as an outcome of interest. Correlations of such proteins with any specific disease score or scale, like the visual analog scale (VAS) [23] for pain or the Fibromyalgia Impact Questionnaire (FIQ), were also recorded.
Quality of Studies
The modified Newcastle–Ottawa scale (NOS) was used for assessing the quality of the included studies by two independent reviewers [24]. The maximum score a study can collect is 9 points. The scale was further adapted according to Nguyen et al. [25] and the Molecular & Cellular Proteomics (MCP) initiative [26] to fit the design and methodology of proteomic studies.
Data Extraction
Two independent researchers (A.G. and S.G.T.) extracted data in a prespecified Excel spreadsheet. Information regarding the study (first author, year, country, funding), the sample (recruitment, number of patients and controls, age and gender of patients and controls, comorbidities, medication), the biological fluid, the fibromyalgia diagnostic criteria, the exclusion criteria and the years of diagnosis, the pain and quality of life scales VAS [23], Pressure Point Threshold (PPT) [27], tender points [28], Widespread Pain Index (WPI) [6], Symptom Severity Scale (SSS) [6], Functional Assessment of Chronic Illness Therapy (FACIT) [29], FIQ [30, 31], Pittsburgh Sleep Quality Index (PSQI) [32], Physical/Mental Component Summary-12 (PCS/MCS-12) [33], Beck Anxiety Inventory (BAI) [34], Beck Depression Inventory (BDI) [35], Hospital Anxiety and Depression Scale (HADS) [36], the methodology (proteomics methodology, database used), and the count and names of proteins were extracted for all included studies.
Data Synthesis
Since a meta-analysis was not feasible due to the existing heterogeneity between biological samples, a systematic synthesis was conducted.
Results
Search Results
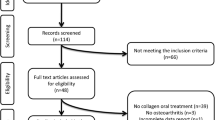
Of the 1099 studies screened, 37 duplicates were removed, and 1062 were reviewed at the title and abstract level. From these, sixty-four studies were reviewed also in full-text form. Four additional studies were identified through citation searching. In total, 10 studies fulfilled the criteria (Table 1) and were included in the present systematic review [37,38,39,40,41,42,43,44, 45•, 46]. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 [20] flowchart is presented in Fig. 1. A list of excluded studies is detailed in Supplementary Table 4.
One study was excluded for examining gut microbiome and serum metabolome in patients with FMS, combined with custom multiplex cytokine and miRNA analysis (FirePlex™ technology) in serum, thus not having relevant results to the present research question [47].
Studies Characteristics
Study Design and Origin
Most studies were conducted in Europe, specifically Italy, Spain, and Sweden [37,38,39,40,41,42,43,44]. The remaining two studies were implemented in Taiwan [45•, 46]. All research was conducted between the years 2009 and 2022, with seven out of ten studies declaring funding sources [37, 38, 40, 41, 44, 45•, 46].
Biological Fluids
Two studies used cerebrospinal fluid (CSF) [41, 42], two saliva [38, 39], four serum samples [37, 43, 45•, 46], while the remaining two used plasma [40, 44]. One of the studies using serum samples involved metabolomics in serum and urine, with a concomitant proteome analysis in serum samples only [45•].
Samples
Patients were recruited through hospitals, Rheumatology and outpatient clinics, and FMS patients’ associations. The sample size ranged from 12 to 39 patients and between 12 to 90 controls, for each study. The present systematic review includes a total of 242 patients with FMS and 297 controls, the latter being either healthy, or pain controls (with inflammatory or non-inflammatory pain).
Regarding biological sex, six studies recruited female participants only [39, 40, 42,43,44, 46], two studies used a mixed-sex sample, with a greater percentage of women [38, 45•], one research item used female patients and mixed-sex controls [41], and the final study failed to provide data regarding participants’ sex [37] (Table 1).
The studies used different exclusion criteria for the selection of participants, including the diagnosis of psychiatric diseases, dementia, epilepsy, alcohol or substance abuse, hypertension, osteoarthritis, use of analgesics, autoimmune diseases, neurological diseases, diabetes mellitus, cardiovascular diseases, pregnancy or childbirth, infectious diseases, active malignant disease, use of immunosuppressants or cortisone, and history of injury. All exclusion criteria used by the studies during sample recruitment are presented in Supplementary Table 5.
FMS Diagnostic Criteria
For the diagnosis of FMS, the researchers utilized different diagnostic criteria in each study (Table 1), with half of the studies using the ACR 1990 [39,40,41,42, 44], two applying the ACR 2010 [43, 46], one the ACR 2016 [37], another the ACR 2011 [45•], and one not specifying which diagnostic criteria were used [38].
Medications
Three of the included studies listed the medications used by the included patients [37, 38, 43]. The most used drugs involved tricyclic antidepressants/amitriptyline, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), analgesics, muscle relaxants, benzodiazepines, and anticonvulsants.
Pain and Quality of Life Scales
Most studies used a scale to quantify patient symptomatology; however, as no common recommendations exist, the selection of such scales was arbitrary. The most frequently used scales included the VAS, which quantifies pain on a level of 1 to 10 and the FIQ, for the assessment of quality of life (QoL) [30, 31, 48, 49]. Patients’ VAS ranged from 5.0 ± 2.2 to 7.9 ± 1.9 (mean ± standard deviation), indicating that they experienced severe pain. Other scales (e.g., PPT, WPI, tender points) were used to quantify pain, while the FIQ, FACIT, PCS-12, MCS-12, and SSS were applied for the QoL [27, 33, 50]. Two research items [38, 46] studied sleep quality using the PSQI scale, with results converging on poor sleep quality, frequent sleep interruptions, and fatigue [32]. Finally, one study [46] focused on quantifying anxiety and depression using the BAI and BDI scales [35, 51], revealing an increased incidence of anxiety and depression in patients with FMS.
Proteomic Methodology and Database
All identified studies involved discovery proteomics, with the majority applying liquid chromatography–mass spectrometry technique for protein analysis. Liquid chromatography–mass spectrometry (LC–MS) consists of a powerful analytical technique that combines the power of LC with the highly sensitive and selective mass resolution capability of mass spectrometry (MS) [52, 53]. This technique was utilized by five of the research groups [40,41,42, 45•, 46]. Four research groups applied 2D electrophoresis (2-DE) techniques. Specifically, two used 2-DE coupled to matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) [39, 43], one used 2-DE/surface-enhanced laser desorption ionization–time of flight mass spectrometry (SELDI-TOF MS) [38], and one applied 2-DE/MS [44] techniques. One research item analyzed the proteins based on a targeted proteomics platform called Olink [37] which enables the analysis of some hundreds of proteins by multiplex assays.
Quality of Included Studies
The summary results regarding the NOS (modified for proteomics studies) score in each included study are presented in Fig. 2. One study achieved the maximum quality score (9) [44], four studies received a total NOS score of 8 [40, 41, 45•, 46], and the remaining scored between 5 and 7 [37,38,39, 42, 43]. The domains with quality concerns for most studies included quantification and the representativeness of controls.
Summary results regarding studies’ quality using the modified Newcastle–Ottawa scale [24]
Proteins Identified
A total of 3328 proteins were identified, including peptides and amino acids. The level of 145 proteins differed significantly between patients with FMS and controls. Based on these studies, higher number of proteins could be identified in CSF (1721 proteins from two studies), followed by plasma (647 proteins, two studies), serum (480 proteins, four studies), and saliva (480 proteins, two studies) samples.
Proteins Identified in Saliva and Their Role
Two studies [38, 39] assessed proteomics in saliva samples and isolated 23 differently expressed proteins in patients with FMS versus controls. One of them [39] used healthy controls, while the other [38] used a mixed sample of a healthy population, patients with rheumatoid arthritis (RA) and patients with migraine. The isolated proteins, the study in which they were identified, the UniProt ID (as the direction of change in their levels, increased or decreased relative to the healthy population) are presented in Table 2. The two studies are in agreement as they both identify transaldolase (TALDO), phosphoglycerate mutase-1 (PGAM1), and calgranulin A (S100-A8) over-expressed in patients with FMS. Additional data regarding fold change and statistical significance are provided in Supplementary Table 6.
TALDO mediates cell shape changes and cell motility and is part of the pentose pathway, associated with NADPH production. As a result, its high levels could be interpreted as the body’s attempt to increase NADPH production to deal with oxidative stress [54]. PGAM1 is an enzyme of the glycolysis pathway, and its elevated levels link FMS to glucose disorders. Additionally, anti-PGAM1 antibodies have been observed in various autoimmune diseases of the CNS [55]. Finally, calgranulin A is a low molecular weight protein that binds calcium and belongs to the S100 protein family, regulating various intracellular processes. An increase in the concentration of calgranulin A is probably associated with oxidative stress [56].
Proteins Identified in the CSF and Their Role
Two different studies published by the same author group performed CSF proteomics [41, 42]. A total of 14 different proteins were identified as being over- or under-expressed in patients with FMS, compared to controls, as shown in Table 3. Additional details are presented in Supplementary Table 7. Proteins identified in more than one study included apolipoprotein C-III, which was also isolated in plasma at low concentrations [44], and complement C4-A, which was observed in high levels at the serum of patients with FMS [46].
Apolipoprotein C-III is an atherogenic protein inhibiting lipoprotein lipase (LPL) activity, displacing LPL from lipid particles. Furthermore, it acts in an inhibitory manner at the triglyceride cycle, leading to higher triglyceride concentrations, which result in arterial stiffness and an increased risk of cardiovascular events [57]. Isolation of apolipoprotein C-III in the CSF has been described in the literature; however, its role in the CNS remains unclear [58]. Complement C4-A is part of the classical complement pathway and is involved in forming the membrane attack complex (MAC), activated by immune complexes and leading to cell lysis [59].
Three additional proteins may play a role in the pathogenesis of FMS: malate dehydrogenase, galectin-binding protein-3 (LG3BP), and ProSAAS (PCKS1). Malate dehydrogenase is involved in gluconeogenesis and energy production for muscle contraction. Changes in protein levels related to glycolysis and gluconeogenesis were also observed in the saliva of patients with FMS [60]. Decreased malate dehydrogenase levels result in low malate concentration; thus, a “Super Malic” dietary supplement has been suggested to have beneficial effects in tampering down FMS symptomatology [61]. It is also worth mentioning that lower levels of malate dehydrogenase have been observed in the synovial fluid of patients with RA, reflecting disturbances in its metabolism [61]. LG3BP is a regulator of pro-inflammatory signaling and is elevated in patients with RA, affecting the balance between pro-inflammatory and anti-inflammatory processes [62]. Finally, PCKS1 is present in the neurons, including processing fetal neuropeptides and inhibiting the enzyme that converts prohormones to their active form [63, 64]. PCKS1 is proteolytically processed and produces various neuropeptides [65]. Thus, elevated PCKS1 concentrations may reflect endocrine disturbances already reported in the literature among patients with FMS [66].
Proteins Identified in Plasma and Their Role
Serum and plasma were studied separately, as they are different biological materials. Plasma is the blood derivative obtained by adding anticoagulant and centrifugation, while serum is the blood derivative that remains after coagulation for 30 min and centrifugation. As a result, differences arise in proteomic and metabolomic studies depending on the biological fluid studied [67]. Two research groups assessed plasma proteomics and reported identifying four common proteins at different levels compared to controls, namely transferrin (TRFE), haptoglobin, α2-macroglobulin, and fibrinogen β chain. TRFE has also been observed at elevated levels in the saliva [38] of patients with FMS. The proteins isolated in plasma and their role are shown in Table 4, while additional details can be found in Supplementary Table 8.
TRFE is the main iron-binding protein. In the inflammatory context of chronic lung disease, the increase in TRFE may reflect an attempt to avoid the deleterious effects of free iron [68]. By binding the free iron, the body is protected from potential damage. Haptoglobin is an acute phase marker and binds to hemoglobin during hemolysis to limit the oxidative properties of heme and allow the complex to be recognized by the macrophage receptor CD163 [69]. It protects the organism from oxidative stress. On the other hand, α2-macroglobulin consists of a protease inhibitor, participating in the immune response by trapping proteases, while it is also involved in the coagulation cascade by inhibiting the anticoagulant effect of protein S [70]. Fibrinogen α, β, and γ chains polymerize and form fibrin, the basic component of the clot. These proteins were isolated in serum and plasma [45•, 46] samples of patients with FMS.
Several isolated proteins are associated with the acute phase reaction, the complement cascade, and coagulation-fibrinolysis. Increased concentrations of TNF-α, IL-8, and IL-10 in FMS have been reported in the literature; however, the data are conflicting as other studies have not confirmed them [16, 71].
Proteins Identified in the Serum and Their Role
Serum was studied by four research teams (Table 5, Supplementary Table 9) [37, 43, 45•, 46]. The proteins identified in the serum of patients with FMS that differed from controls and were reported by most studies included fibrinogen A chain and profilin-1 (Table 5). Proteins identified in the serum and plasma involved A, B, and C chain of fibrinogen, serum amyloid (P-component, protein A4), C1qC, and thrombospondin-1. Calgranulin C and the immunoglobin Ig lambda-2 chain C region were identified in both the serum and saliva of patients with FMS. Finally, a common protein between serum and CSF was complement C4-A.
Profilin-1 is a protein that regulates actin dynamics in cells by promoting actin filament assembly and turnover, essential for processes like cell motility, shape maintenance, and endocytosis. It is crucial in facilitating cellular movement and maintaining cell structure [72, 73]. The serum amyloid p-component reduces neutrophil adhesion to proteins, inhibits the differentiation of monocytes into fibrocytes, attenuates profibrotic macrophages, activates the complement pathway, and promotes phagocytosis of cell debris. These effects regulate key aspects of inflammation and set a threshold for immune cell activation [74,75,76]. Serum amyloid A4 is an acute phase reactant with a procoagulant role [77]. Finally, thrombospondins have diverse tissue-specific actions that include effects on angiogenesis, platelet activation, inflammation, and cell death that directly impact wound healing and tumorigenesis [78, 79].
Proteins Identified in Several Biological Fluids
More than one study identified 20 proteins differentially expressed in patients with FMS compared to controls. Specifically, fibrinogen α, β, and γ chains were identified in serum and plasma samples. These proteins play a key role in the coagulation cascade, polymerize, and create fibrin. However, their levels varied between studies. TRFE, the major iron-binding protein, was elevated in plasma and saliva samples, while profilin-1 was detected in three studies with decreased concentrations in serum and higher levels in saliva samples of patients with FMS. Interestingly, one investigator [45•] characterized profilin-1 as a discriminating marker between two subgroups of FMS, experiencing pain and stiffness as prominent symptoms, respectively.
C4-A concentrations were elevated in serum and CSF samples, and C1qC was observed in high levels in the plasma and serum samples of patients with FMS. A2-macroglobulin and serum amyloid (A4 and p-component) were isolated in plasma and serum, while haptoglobin was identified in plasma samples. Similarly, thrombospondin-1 was identified in plasma and serum samples. Elevated PGAM1, TALDO, and calgranulin A levels were observed in saliva samples of patients with FMS. Calgranulin C was over-expressed in serum and saliva of patients with FMS, while several immunoglobulin fractions were isolated in plasma, serum, and saliva samples. All proteins identified as being differentially expressed in FMS compared to controls in more than one study are presented in Table 6.
Association of Proteins with Scales of Disease Severity and Quality of Life
Bazzichi et al. [39] assessed the association between elevated saliva TALDO and PGAM1 levels and pain (VAS), QoL (FIQ), and the number of tender points. However, no significant association was detected. Similarly, Ciregia et al. [38] failed to observe any association between isolated proteins in saliva and pain (VAS), QoL (FIQ, FACIT), or tender points count.
Han et al. [46] found a negative correlation between serum keratin (Keratin, type II cytoskeletal 80) and depressive symptoms (using the BDI scale) (P = 0.014, r = − 0.567) and a mild correlation between the GHV1-46 immunoglobulin segment (Ig heavy chain V-I region HG3) and pain (using the VAS scale, P = 0.049, r = 0.470).
Wahlen et al. [44] correlated elevated α2-macroglobulin and plasma TRFE concentrations to moderate-to-severe pain intensity. On the other hand, Ruggiero et al. [43] failed to report any associations between isolated serum proteins, disease duration, and pain (VAS) and QoL (FIQ) scales. Finally, Hsu et al. [45•] attempted to discriminate pain and soreness phenotypes in patients with FMS into two groups, focusing on the predominant reported symptom (pain or stiffness), concluding that this allocation is possible using proteomics, as each group exhibits different concentrations of proteomic markers.
Discussion
The results presented herein reveal that the identified proteins with significant alterations in the biological fluids of patients with FMS are mainly related to the immune system, the complement cascade, coagulation, and fibrinolysis.
Iron Metabolism Biomarkers
The most prevalent protein isolated in the included studies involved TRFE, over-expressed in plasma and saliva in FMS compared to healthy and pain controls. Since TRFE is an iron-binding protein, it reflects the body’s need for iron supply [80]. The association of FMS and iron metabolism disorders has been reported, with a possible mechanism being the involvement of iron in the production of dopamine and serotonin as a cofactor [81]. Elevated TRFE concentrations have been observed in the CSF of patients with restless legs syndrome [82, 83]. However, in a case–control study, no difference was observed between patients with FMS and controls regarding serum iron, TRFE, and ferritin levels [84]. Recently, a Taiwan nationwide study [85] revealed that adults with iron deficiency anemia had increased chances of developing FMS. An additional piece in the puzzle was provided by an intervention study [86] conducted on women with FMS and low ferritin levels, who had previously failed to improve iron status with oral supplements. When intravenous iron (ferric carboxymaltose) treatment was initiated, a reduction in pain sensation and an improvement in QoL were noted [86]. Similar results were also reported from a blind, randomized, placebo-controlled trial [87], indicating a role for iron, in FMS treatment among patients with low iron stores.
Coagulation Biomarkers
Concentrations of fibrinogen α, β, and γ chains differed between patients and controls in serum and plasma samples, but findings regarding the direction of difference varied among investigators. Research is consistent with the elevated fibrinogen levels among patients with FMS, indicative of a prothrombotic state [88]. Complement C1QC and C4A were also elevated in various biological fluids (CSF, plasma, serum) in FMS. These proteins are involved in the formation of MAC through the classical complement pathway, which is stimulated by IgM/IgG immune complexes and leads to chemotaxis and mobilization of cell opsonization [59, 89]. Certain coagulation proteins can also activate the complement cascade [90]. These findings are consistent with the involvement of the coagulation cascade and complement in the pathophysiology of FMS.
Inflammation Biomarkers
Inflammation is a known driver of FMS. The increased amounts of skin mast cells [91, 92], substance P, and corticotropin-releasing hormone (CRH) have been observed in FMS [93, 94], appear to activate the release of IL-8 and monocyte chemoattractant protein-1 (MCP-1, a pro-inflammatory chemokine, member of a subfamily of the IL-8 supergene family) [95], elevating plasma concentrations [96]. As a result, both IL-8 and MCP-1 have been suggested as possible diagnostic biomarkers for FMS, conferring an inflammatory action [97]. At the proteomics level, different concentrations of thrombospondin-1 were observed in the serum and plasma of patients with FMS compared to controls. Inflammation in the CNS has been suggested as a mechanism involved in the pathogenesis of FMS, as impaired coagulation and fibrinolysis have been associated with degeneration of the CNS [98]. The processing of pain is transmitted from peripheral tissues to the brain and is influenced by a variety of endogenous and exogenous processes [96], including impaired coagulation and fibrinolysis [98]. Disturbances in any point in these pathways have been also observed in proteomic studies among patients with multiple sclerosis [99]. In parallel, small-fiber neuropathy (SFN) has been an additional consistent finding in FMS [100,101,102], indicative of CNS degeneration. However, more research is required to clarify the association between neuroinflammation and FMS.
As for the possible therapeutic component of anti-inflammatory regimes, research has revealed that the consumption of refined olive oil (ROO) among patients with FMS reduced fibrinogen levels, platelet distribution width, neutrophil-to-lymphocyte ratio, and erythrocyte sedimentation rate (ESR) concentrations [103].
Oxidative Stress Biomarkers
Haptoglobin is an acute phase protein with antioxidant activity that binds hemoglobin and prevents the toxic effect of iron. Elevated haptoglobin levels were recorded in the plasma of patients with FMS as a possible coping mechanism to mitigate oxidative stress [104]. Conventional analytical methods also verify the existence of higher plasma haptoglobin levels in FMS and associate them with symptoms of depression, hyperalgesia, exhaustion, and sleep disturbances [105]. Recent research suggests increased oxygen free radicals among patients with FMS [106]. In more detail, prooxidative factors such as nitric oxide, products of free radical lipid peroxidation, including serum malondialdehyde and lipid hydroperoxide demonstrate an increased concentration, whereas xanthine oxidase levels are decreased, a process commonly occurring when oxygen free radicals are produced [106,107,108,109]. In parallel, the levels of endogenous antioxidants are reduced, including glutathione, superoxide dismutase, and total antioxidant status in serum, as a response to the elevated oxidative stress [106, 110]. This phenomena have led to several researchers arguing whether FMS is actually an oxidative stress–driven disorder [107], since greater levels of prooxidative factors and mitophagy appear to augment pain sensitization [109]. Interestingly, a recent systematic review concluded that supplementation with antioxidant vitamins and coenzyme Q10 for at least 6 weeks was associated with a reduced pain perception in 80% of the patients with FMS [111], indicating that antioxidants appear to have an analgesic role in FMS management [111, 112].
TALDO is an enzyme of the pentose pathway that leads to NADPH production [54, 113]. Over-expression of TALDO was recorded in the saliva of patients with FMS, and this increase may also reflect the body’s attempt to compensate for the increased oxidative stress endured. Although higher TALDO levels were observed in FMS compared with the healthy population, no differences were noted between FMS and patients with RA, or migraine.
Compared to healthy controls, patients with FMS demonstrated increased calgranulin A (S100-A8), but when compared to patients with RA and migraine, this difference ceased to exist. On the other hand, calgranulin C (S100-A12) concentrations were elevated in the saliva and serum of patients with FMS. Calgranulins are homogeneous low molecular weight calcium-binding proteins belonging to the S100 protein family. They are involved in various cellular responses and intracellular pathways that regulate cell differentiation, cytoskeleton, structural organization of membranes, intracellular calcium homeostasis, and protection against oxidative stress [114]. It is worth highlighting another study, not included in the present systematic review, published in Italian, which verified elevated calgranulin A and C saliva concentrations in patients with FMS [115].
Research indicates that the consumption of extra-virgin olive oil (EVOO) acts protectively in balancing redox homeostasis and tampering down inflammation [116, 117]. This is mostly due to the phenolic content of EVOO, and in particular hydroxy-tyrosol (HT). Interestingly, a preliminary study [118] assessed the effect of a high-HT nutritional treatment to the proteome of dermal fibroblasts of a single patient with FMS, versus a healthy control. The results revealed that treatment with HT normalized the differential expression in proteins involved in the turnover of extracellular matrix and oxidative metabolism, observed in the patient with FMS, against the healthy control [118]. Although greatly underpowered, this study highlighted a possible therapeutic pathway for FMS, in need of more high-quality research. Other research groups have also identified EVOO as an important adjuvant in FMS treatment. In a RCT [119], women with FMS treated with 50 mL of EVOO or other ROO for a period of 3 weeks showed improvements in their antioxidative profiles (protein carbonyls, lipid peroxidation) and pain (FIQ), and QoL scales, exhibiting antithrombotic and anti-inflammatory properties [103].
Immune Response Biomarkers
Serum amyloid (P component) was elevated in plasma and serum, while serum amyloid (SA4) was high in plasma and low in serum. These proteins belong to the acute phase proteins and regulate the immune response. Different saliva and plasma concentrations of Ig kappa chain C region and Ig alpha-1 chain C region immunoglobulin segments were observed in patients with FMS. In parallel, Ig lambda-2 chain C region segments were high in saliva and low in serum samples. To date, the exact role of the immune system in pain development and sensation remains unclear [120]. Nonetheless, an increased incidence of immunodeficiency has been observed in patients with FMS, and conversely, FMS tends to be more common in patients with primary immunodeficiency [121, 122]. Furthermore, immune aberrations have been reported in FMS, which were considered partially responsible for the sensation of pain [123•].
PGAM1 is an enzyme of glycolysis, and serum autoantibodies against PGAM1 have been reported in autoimmune hepatitis and various neurological diseases, including multiple sclerosis and neuromyelitis optica [55, 124]. As seen in the present results, PGAM1 concentrations were also increased in the saliva of patients with FMS. As the salivary gland innervates directly from the trigeminovascular system, saliva contains several neuropeptides, potentially providing information regarding CNS pathology and related disorders [125, 126]. In this manner, the elevated PGAM1 saliva levels could be of particular interest, as FMS has been associated with several neurological disorders [127]. More research is required to delineate this association.
Cardiovascular Risk Biomarkers
Profilin-1 is an actin-binding protein that regulates DNA damage response and repair mechanisms [73]. Saliva samples of patients with FMS exhibited higher profilin-1 concentrations than controls, whereas lower profilin-1 concentrations were observed in serum samples. In parallel, it has been suggested [45•] that profilin-1 could identify patients with FMS, depending on the main reported symptom (pain or stiffness). Recently, profilin-1 emerged as a new player in the field of atherosclerosis; it is accumulated in high concentrations in stable atherosclerotic plaques and thrombi from infarct-related arteries in cases of acute myocardial infarction [128]. Furthermore, several studies have reported histological abnormalities in the muscle tissue of patients with FMS, indicative of microvascular dysfunction, including capillary dysfunction and myocyte mitochondric abnormalities [106, 129,130,131].
Finally, different apolipoprotein C-III concentrations were reported in the plasma and CSF of patients with FMS. Apolipoprotein C-III is an atherogenic protein that leads to higher triglyceride levels, resulting in augmented risk of cardiovascular events. Its role in the CNS remains unclear. Higher serum total cholesterol, LDL, and triglyceride levels have been observed in women with FMS, and these patients are known to have increased cardiovascular risk in parallel to obesity [132,133,134].
Other Biomarkers with Multiple Roles
A common protease inhibitor involved in the coagulation cascade, inflammation, and autoimmunity phenomena is α2-macroglobulin [135]. Plasma levels of α2-macroglobulin were elevated in patients with FMS compared with healthy controls. A2-macroglobulin has been detected in the proteome of patients with chronic fatigue syndrome [136] and multiple sclerosis [137]. Interestingly, new research and advances in pain management have suggested using α2-macroglobulin for treating neuropathic pain [138•, 139]. As a result, α2-macroglobulin injections are frequently applied for managing knee osteoarthritis [140], as they prevent cartilage degeneration by inhibiting catabolic enzymes and cytokines [141].
Associations Between Proteomics and QoL of Patients
The present systematic review aimed to identify potential relationships between specific proteomic markers in FMS and QoL scales; however, for the most part, the results were inconclusive. One study using plasma samples [44] associated elevated α2-macroglobulin and TRFE with moderate and severe pain intensity. Finally, another study using serum samples [45•] concluded that different clinical profiles may be associated with different proteomic markers.
Combining Biomarkers for Diagnosis and Treatment
The quest for diagnostic biomarkers for FMS continues, aiming to provide a critical step towards prompt intervention [96]. Annemans [22] revealed that establishing an FMS diagnosis decreases the financial costs associated with tests and imaging, referrals, doctor visits, and pharmaceuticals, showing that a diagnosis reduces the use of resources. Thus, efforts to identify and combine diagnostic markers are warranted to reduce the burden and costs associated with FMS. It is important to note, that although several different proteins were identified herein, it is difficult to understand if the same biological pathways act as triggers for the development of FMS, or if different pathways result in the same disease phenotype for different patients. Thus, we are currently unsure which combination of biomarkers can offer improved diagnostic ability for FMS, or whether a combination therapy (antioxidants, iron, antithrombotic, etc.) conferring a comprehensive synergistic action can tamper down FMS symptoms. What is known though from an early study [142], is that approximately 30% of patients with FMS have reported taking dietary supplements or making holistic dietary changes in response to their disease, according to their healthcare professionals, all achieving improved pain relief. As for the diagnosis, one of the studies [46] included herein reported using a decision tree model to differentiate patients with FMS and controls based on the expression levels of histidine protein methyltransferase 1 homolog (HMPT1), Interleukin-1 receptor accessory protein (IL1RAP) and Ig lambda chain V-IV region (IGL3-25), yielding an accuracy of up to 0.97. No other efforts to achieve improved diagnostic accuracy were reported by the authors of the remaining proteomics studies, and as quantitative data were not included in all research, we were unable to conduct this either.
Limitations of the Included Studies
Some of the included studies shared common authors. Specifically, the two studies using CSF samples [41, 42] had the same first author and two additional authors in common. Also, the two studies investigating proteomics in saliva [38, 39] were conducted by the same research group core, sharing five common authors, with one study essentially being the extension of the other, as it included a healthy population and a group of patients with chronic pain, as controls. This could be considered a confounding factor.
Most research items failed to report the medications taken by the patients or controls. Drugs can affect the proteome, as they have various actions on the metabolism and excretion of proteins from the body.
Finally, there were great differences in the proteomics methodologies among the studies. Distinct methods have varying sensitivity and specificity and may produce different results in the proteins detected and quantified. Each method has its advantages and limitations and may excel in different areas of proteomic research. Finally, the inherent differences in sample preparation, separation, and detection can lead to variations in the total number of proteins detected.
Conclusions
In summary, proteomics consist of a useful tool, providing insight into the processes and signaling pathways that may be involved in the pathogenesis of FMS. In the present systematic review, the proteome of patients with FMS was studied, and specific protein expression patterns were identified. Proteins related to the complement cascade, the coagulation cascade, inflammation, the immune system, iron metabolism, and the oxidative stress process were found to be dysregulated in FMS patients. FMS appears closely related to the oxidative stress pathway, as many proteins protecting the body from oxidative stress appear are dysregulated. However, more primary studies are required to aid our understanding of this association.
Data Availability
Extracted data for this review are available upon request to the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mueller C, Fang Y-HD, Jones C, McConathy JE, Raman F, Lapi SE, et al. Evidence of neuroinflammation in fibromyalgia syndrome: a [18F]DPA-714 positron emission tomography study. Pain. 2023;164:2285–95.
Beiner E, Lucas V, Reichert J, Buhai D-V, Jesinghaus M, Vock S, et al. Stress biomarkers in individuals with fibromyalgia syndrome: a systematic review with meta-analysis. Pain. 2023;164:1416–27.
Bair MJ, Krebs EE. Fibromyalgia. Ann Intern Med. 2020;172:ITC33–48.
Cabo-Meseguer A, Cerdá-Olmedo G, Trillo-Mata JL. Fibromyalgia: prevalence, epidemiologic profiles and economic costs. Med Clin (Barc). 2017;149:441–8.
Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int. 2017;37:1527–39.
Wolfe F, Häuser W. Fibromyalgia diagnosis and diagnostic criteria. Ann Med. 2011;43:495–502.
Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–72.
Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RL, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46:319–29.
Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–22.
Alharbi RA. Proteomics approach and techniques in identification of reliable biomarkers for diseases. Saudi J Biol Sci. 2020;27:968–74.
Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–29.
Lerma C, Martinez A, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: correlation with symptoms severity. Arthritis Res Ther. 2011;13:R185.
Nicholl BI, Holliday KL, Macfarlane GJ, Thomson W, Davies KA, O’Neill TW, et al. Association of HTR2A polymorphisms with chronic widespread pain and the extent of musculoskeletal pain: results from two population-based cohorts. Arthritis Rheum. 2011;63:810–8.
Peck MM, Maram R, Mohamed A, Ochoa Crespo D, Kaur G, Ashraf I, et al. The influence of pro-inflammatory cytokines and genetic variants in the development of fibromyalgia: a traditional review. Cureus. 2020;12: e10276.
Riva R, Mork PJ, Westgaard RH, Okkenhaug Johansen T, Lundberg U. Catecholamines and heart rate in female fibromyalgia patients. J Psychosom Res. 2012;72:51–7.
Amel Kashipaz MR, Swinden D, Todd I, Powell RJ. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clin Exp Immunol. 2003;132:360–5.
Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, Kushner I, et al. The fibromyalgia family study: a genome-wide linkage scan study. Arthritis Rheum. 2013;65:1122–8.
Patel S, Ling J, Kim SJ, Schey KL, Rose K, Kuchtey RW. Proteomic analysis of macular fluid associated with advanced glaucomatous excavation. JAMA Ophthalmol. 2016;134:108–10.
Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics: technologies and their applications. J Chromatogr Sci. 2017;55:182–96.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:I6890.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Kelly A-M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–7.
Wells GA, Shea Beverley, O’Connell Dianne, Peterson Joan, Welch Vivian, Losos Michael, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ontario, Canada; 2000.
Nguyen TPH, Patrick CJ, Parry LJ, Familari M. Using proteomics to advance the search for potential biomarkers for preeclampsia: a systematic review and meta-analysis. PLoS ONE. 2019;14: e0214671.
Celis JE, Carr SA, Bradshaw RA. New guidelines for clinical proteomics manuscripts. Mol Cell Proteomics. 2008;7:2071–2.
Park G, Kim CW, Park SB, Kim MJ, Jang SH. Reliability and usefulness of the pressure pain threshold measurement in patients with myofascial pain. Ann Rehabil Med. 2011;35:412–7.
Lundberg G, Gerdle B. Tender point scores and their relations to signs of mobility, symptoms, and disability in female home care personnel and the prevalence of fibromyalgia syndrome. J Rheumatol. 2002;29:603–13.
Webster K, Cella D, Yost K. The F unctional A ssessment of C hronic I llness T herapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79.
Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–33.
Williams DA, Arnold LM. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ). Arthritis Care Res (Hoboken). 2011;63(Suppl 1):S86–97.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:205031211667172.
Lykke J, Hesse M, Austin SF, Oestrich I. Validity of the BPRS, the BDI and the BAI in dual diagnosis patients. Addict Behav. 2008;33:292–300.
Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100.
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2022;52:69–77.
Fineschi S, Klar J, Gustafsson KA, Jonsson K, Karlsson B, Dahl N. Inflammation and interferon signatures in peripheral B-lymphocytes and sera of individuals with fibromyalgia. Front Immunol. 2022;13: 874490.
Ciregia F, Giacomelli C, Giusti L, Boldrini C, Piga I, Pepe P, et al. Putative salivary biomarkers useful to differentiate patients with fibromyalgia. J Proteomics. 2019;190:44–54.
Bazzichi L, Ciregia F, Giusti L, Baldini C, Giannaccini G, Giacomelli C, et al. Detection of potential markers of primary fibromyalgia syndrome in human saliva. Proteomics Clin Appl. 2009;3:1296–304.
Ramírez-Tejero JA, Martínez-Lara E, Rus A, Camacho MV, Del Moral ML, Siles E. Insight into the biological pathways underlying fibromyalgia by a proteomic approach. J Proteomics. 2018;186:47–55.
Khoonsari PE, Musunri S, Herman S, Svensson CI, Tanum L, Gordh T, et al. Systematic analysis of the cerebrospinal fluid proteome of fibromyalgia patients. J Proteomics. 2019;190:35–43.
Khoonsari PE, Ossipova E, Lengqvist J, Svensson CI, Kosek E, Kadetoff D, et al. The human CSF pain proteome. J Proteomics. 2019;190:67–76.
Ruggiero V, Era B, Cacace E, Molin L, Corda M, Fais A, et al. A preliminary study on serum proteomics in fibromyalgia syndrome. Clin Chem Lab Med. 2014;52:e207–210.
Wåhlén K, Ernberg M, Kosek E, Mannerkorpi K, Gerdle B, Ghafouri B. Significant correlation between plasma proteome profile and pain intensity, sensitivity, and psychological distress in women with fibromyalgia. Sci Rep. 2020;10:12508.
• Hsu W, Han D, Ku W, Chao Y, Chen C, Lin Y. Metabolomic and proteomic characterization of sng and pain phenotypes in fibromyalgia. Eur J Pain. 2022;26:445–62. The study identified potential biomarkers from FM patients that could discriminate sng and pain phenotypes in patients with FMS.
Han C-L, Sheng Y-C, Wang S-Y, Chen Y-H, Kang J-H. Serum proteome profiles revealed dysregulated proteins and mechanisms associated with fibromyalgia syndrome in women. Sci Rep. 2020;10:12347.
Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G, Abecia L, Lavín JL, van Liempd S, et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine. 2019;46:499–511.
Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–8.
Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF. Arthritis Care Res (Hoboken). 2011;63:S240–52.
Karcioglu O, Topacoglu H, Dikme O, Dikme O. A systematic review of the pain scales in adults: which to use? Am J Emerg Med. 2018;36:707–14.
Steer RA, Ranieri WF, Beck AT, Clark DA. Further evidence for the validity of the beck anxiety inventory with psychiatric outpatients. J Anxiety Disord. 1993;7:195–205.
Beynon RJ. The dynamics of the proteome: strategies for measuring protein turnover on a proteome-wide scale. Brief Funct Genomic Proteomic. 2005;3:382–90.
Chandramouli K, Qian P-Y. Proteomics: challenges, techniques and possibilities to overcome biological sample complexity. Hum Genomics Proteomics. 2009;2009: 239204.
Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med. 2011;17:395–403.
Kimura A, Sakurai T, Koumura A, Yamada M, Hayashi Y, Tanaka Y, et al. High prevalence of autoantibodies against phosphoglycerate mutase 1 in patients with autoimmune central nervous system diseases. J Neuroimmunol. 2010;219:105–8.
Hu W, Lin C. S100a8 silencing attenuates inflammation, oxidative stress and apoptosis in BV2 cells induced by oxygen-glucose deprivation and reoxygenation by upregulating GAB1 expression. Mol Med Rep. 2021;23:64.
Ramms B, Gordts PLSM. Apolipoprotein C-III in triglyceride-rich lipoprotein metabolism. Curr Opin Lipidol. 2018;29:171–9.
Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci USA. 1979;76:4646–9.
Wang H, Liu M. Complement C4, infections, and autoimmune diseases. Front Immunol. 2021;12: 694928.
Minárik P, Tomásková N, Kollárová M, Antalík M. Malate dehydrogenases–structure and function. Gen Physiol Biophys. 2002;21:257–65.
Russell IJ, Michalek JE, Flechas JD, Abraham GE. Treatment of fibromyalgia syndrome with Super Malic: a randomized, double blind, placebo controlled, crossover pilot study. J Rheumatol. 1995;22:953–8.
Ohshima S, Kuchen S, Seemayer CA, Kyburz D, Hirt A, Klinzing S, et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003;48:2788–95.
Lanoue E, Day R. Coexpression of proprotein convertase SPC3 and the neuroendocrine precursor proSAAS. Endocrinology. 2001;142:4141–9.
Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, Chrétien M, et al. Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J Biol Chem. 2001;276:32720–8.
Mzhavia N, Berman Y, Che FY, Fricker LD, Devi LA. ProSAAS processing in mouse brain and pituitary. J Biol Chem. 2001;276:6207–13.
Valença MM, Medeiros FL, Martins HA, Massaud RM, Peres MFP. Neuroendocrine dysfunction in fibromyalgia and migraine. Curr Pain Headache Rep. 2009;13:358–64.
Liu X, Hoene M, Wang X, Yin P, Häring H-U, Xu G, et al. Serum or plasma, what is the difference? Investigations to facilitate the sample material selection decision making process for metabolomics studies and beyond. Anal Chim Acta. 2018;1037:293–300.
Baralla A, Fois AG, Sotgiu E, Zinellu E, Mangoni AA, Sotgia S, et al. Plasma proteomic signatures in early chronic obstructive pulmonary disease. Proteomics Clin Appl. 2018;12:1700088.
Andersen CBF, Stødkilde K, Sæderup KL, Kuhlee A, Raunser S, Graversen JH, et al. Haptoglobin. Antioxid Redox Signal. 2017;26:814–31.
Cvirn G, Gallistl S, Koestenberger M, Kutschera J, Leschnik B, Muntean W. Alpha 2-macroglobulin enhances prothrombin activation and thrombin potential by inhibiting the anticoagulant protein C/protein S system in cord and adult plasma. Thromb Res. 2002;105:433–9.
Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25:225–30.
Allen A, Gau D, Roy P. The role of profilin-1 in cardiovascular diseases. J Cell Sci. 2021;134:jcs249060.
Lee C-J, Yoon M-J, Kim DH, Kim TU, Kang Y-J. Profilin-1; a novel regulator of DNA damage response and repair machinery in keratinocytes. Mol Biol Rep. 2021;48:1439–52.
de Haas CJ. New insights into the role of serum amyloid P component, a novel lipopolysaccharide-binding protein. FEMS Immunol Med Microbiol. 1999;26:197–202.
Cox N, Pilling D, Gomer RH. Serum amyloid P: a systemic regulator of the innate immune response. J Leukoc Biol. 2014;96:739–43.
Verwey NA, Schuitemaker A, van der Flier WM, Mulder SD, Mulder C, Hack CE, et al. Serum amyloid P component as a biomarker in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;26:522–7.
Fernández JA, Deguchi H, Elias DJ, Griffin JH. Serum amyloid A4 is a procoagulant apolipoprotein that it is elevated in venous thrombosis patients. Res Pract Thromb Haemost. 2020;4:217–23.
Kale A, Rogers NM, Ghimire K. Thrombospondin-1 CD47 signalling: from mechanisms to medicine. Int J Mol Sci. 2021;22:4062.
Iruela-Arispe ML, Luque A, Lee N. Thrombospondin modules and angiogenesis. Int J Biochem Cell Biol. 2004;36:1070–8.
Baird-Gunning J, Bromley J. Correcting iron deficiency. Aust Prescr. 2016;39:193–6.
Ortancil O, Sanli A, Eryuksel R, Basaran A, Ankarali H. Association between serum ferritin level and fibromyalgia syndrome. Eur J Clin Nutr. 2010;64:308–12.
Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–700.
Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14:43–7.
Mader R, Koton Y, Buskila D, Herer P, Elias M. Serum iron and iron stores in non-anemic patients with fibromyalgia. Clin Rheumatol. 2012;31:595–9.
Yao WC, Chen HJ, Leong KH, Chang KL, Wang YTT, Wu LC, et al. The risk of fibromyalgia in patients with iron deficiency anemia: a nationwide population-based cohort study. Sci Rep. 2021;11:10496.
Hamarat H, Gürcü S, Kıvanç BK, Aydemir AE. Ferric carboxymaltose therapy reduces pain and improves the quality of life in female patients with fibromyalgia. Eur Rev Med Pharmacol Sci. 2023;27:10375–80.
Boomershine CS, Koch TA, Morris D. A blinded, randomized, placebo-controlled study to investigate the efficacy and safety of ferric carboxymaltose in iron-deficient patients with fibromyalgia. Rheumatol Ther. 2018;5:271–81.
Molina F, del Moral ML, La Rubia M, Blanco S, Carmona R, Rus A. Are patients with fibromyalgia in a prothrombotic state? Biol Res Nurs. 2019;21:224–30.
Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97.
Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement–their role in inflammation. Semin Immunopathol. 2012;34:151–65.
Blanco I, Béritze N, Argüelles M, Cárcaba V, Fernández F, Janciauskiene S, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403–12.
Eneström S, Bengtsson A, Frödin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia–relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308–13.
McLean SA, Williams DA, Stein PK, Harris RE, Lyden AK, Whalen G, et al. Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology Neuropsychopharmacology. 2006;31:2776–82.
Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593–601.
Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705.
Hackshaw KV. The search for biomarkers in fibromyalgia. Diagnostics. 2021;11:156.
Ang DC, Moore MN, Hilligoss J, Tabbey R. MCP-1 and IL-8 as pain biomarkers in fibromyalgia: a pilot study. Pain Med. 2011;12:1154–61.
Bardehle S, Rafalski VA, Akassoglou K. Breaking boundaries-coagulation and fibrinolysis at the neurovascular interface. Front Cell Neurosci. 2015;9:354.
Han MH, Hwang S-I, Roy DB, Lundgren DH, Price JV, Ousman SS, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–81.
Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154:2310–6.
Levine TD, Saperstein DS, Levine A, Hackshaw K, Lawson V. Small fiber neuropathy in patients meeting diagnostic criteria for fibromyalgia. J Neurol Disord. 2016;4:305.
Lawson VH, Grewal J, Hackshaw KV, Mongiovi PC, Stino AM. Fibromyalgia syndrome and small fiber, early or mild sensory polyneuropathy. Muscle Nerve. 2018;58:625–30.
Rus A, Molina F, Martínez-Ramírez MJ, Aguilar-Ferrándiz ME, Carmona R, Del MML. Effects of olive oil consumption on cardiovascular risk factors in patients with fibromyalgia. Nutrients. 2020;12:918.
Bertaggia E, Scabia G, Dalise S, Lo Verso F, Santini F, Vitti P, et al. Haptoglobin is required to prevent oxidative stress and muscle atrophy. PLoS ONE. 2014;9: e100745.
Maes M, Scharpé S, Meltzer HY, Cosyns P. Relationships between increased haptoglobin plasma levels and activation of cell-mediated immunity in depression. Biol Psychiatry. 1993;34:690–701.
Coderre TJ. Contribution of microvascular dysfunction to chronic pain. Front Pain Res. 2023;4:1111559.
Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, et al. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int. 2005;25:188–90.
Ozgocmen S, Ozyurt H, Sogut S, Akyol O, Ardicoglu O, Yildizhan H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: etiologic and therapeutic concerns. Rheumatol Int. 2006;26:598–603.
Assavarittirong C, Samborski W, Grygiel-Górniak B. Oxidative stress in fibromyalgia: from pathology to treatment. Oxid Med Cell Longev. 2022;2022:1582432.
Sendur OF, Turan Y, Tastaban E, Yenisey C, Serter M. Serum antioxidants and nitric oxide levels in fibromyalgia: a controlled study. Rheumatol Int. 2009;29:629–33.
Fernández-Araque A, Verde Z, Torres-Ortega C, Sainz-Gil M, Velasco-Gonzalez V, González-Bernal JJ, et al. Effects of antioxidants on pain perception in patients with fibromyalgia—a systematic review. J Clin Med. 2022;11:2462.
Elkholy NS, Mohammed HS, Shafaa MW. Assessment of the therapeutic potential of lutein and beta-carotene nanodispersions in a rat model of fibromyalgia. Sci Rep. 2023;13:1–18.
Samland AK, Sprenger GA. Transaldolase: from biochemistry to human disease. Int J Biochem Cell Biol. 2009;41:1482–94.
Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69:986–94.
Giacomelli C, Bazzichi L, Giusti L, Ciregia F, Baldini C, Da Valle Y, et al. MALDI-TOF and SELDI-TOF analysis: “tandem” techniques to identify potential biomarker in fibromyalgia. Reumatismo. 2011;63:165–70.
Derakhshandeh-Rishehri SM, Kazemi A, Shim SR, Lotfi M, Mohabati S, Nouri M, et al. Effect of olive oil phenols on oxidative stress biomarkers: a systematic review and dose–response meta-analysis of randomized clinical trials. Food Sci Nutr. 2023;11:2393–402.
George ES, Marshall S, Mayr HL, Trakman GL, Tatucu-Babet OA, Lassemillante ACM, et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2019;59:2772–95.
Ramírez-Tejero JA, Martínez-Lara E, Peinado MÁ, Del MML, Siles E. Hydroxytyrosol as a promising ally in the treatment of fibromyalgia. Nutrients. 2020;12:2386.
Rus A, Molina F, Ramos MM, Martínez-Ramírez MJ, del Moral ML. Extra virgin olive oil improves oxidative stress, functional capacity, and health-related psychological status in patients with fibromyalgia: a preliminary study. Biol Res Nurs. 2017;19:106–15.
Totsch SK, Sorge RE. Immune system involvement in specific pain conditions. Mol Pain. 2017;13:1744806917724559.
Barton JC, Bertoli LF, Barton JC, Acton RT. Fibromyalgia in 300 adult index patients with primary immunodeficiency. Clin Exp Rheumatol. 2017;35(Suppl 1):68–73.
Caro XJ, Winter EF. Unexpectedly high prevalence of primary immune deficiency in fibromyalgia: serologic features and clinical correlates. Clin Exp Rheumatol. 2022;40(6):1076–83.
• Björkander S, Ernberg M, Bileviciute-Ljungar I. Reduced immune system responsiveness in fibromyalgia - a pilot study. Clin Immunol Commun. 2022;2:46–53. This pilot study shows evidence that the presence of immune aberrations in FMS is at least partially responsible for the associated pain sensation.
Sakurai T, Kimura A, Yamada M, Koumura A, Hayashi Y, Tanaka Y, et al. Identification of antibodies as biological markers in serum from multiple sclerosis patients by immunoproteomic approach. J Neuroimmunol. 2011;233:175–80.
Nam JH, Lee HS, Kim J, Kim J, Chu MK. Salivary glutamate is elevated in individuals with chronic migraine. Cephalalgia. 2018;38:1485–92.
Hagedorn JM, Gunn J, Budwany R, D’souza RS, Chakravarthy K, Deer TR. How well do current laboratory biomarkers inform clinical decision-making in chronic pain management? J Pain Res. 2021;14:3695–710.
Watson NF, Buchwald D, Goldberg J, Noonan C, Ellenbogen RG. Neurological signs and symptoms in fibromyalgia. Arthritis Rheum. 2009;60:2844.
Paszek E, Zajdel W, Rajs T, Żmudka K, Legutko J, Kleczyński P. Profilin 1 and mitochondria—partners in the pathogenesis of coronary artery disease? Int J Mol Sci. 2021;22:1–16.
Kalyan-Raman UP, Kalyan-Raman K, Yunus MB, Masi AT. Muscle pathology in primary fibromyalgia syndrome: a light microscopic, histochemical and ultrastructural study. J Rheumatol. 1984;11:808–13.
Drewes AM, Andreasen A, Schrøder HD, Høgsaa B, Jennum P. Pathology of skeletal muscle in fibromyalgia: a histo-immuno-chemical and ultrastructural study. Br J Rheumatol. 1993;32:479–83.
Grassi W, Core P, Carlino G, Salaffi F, Cervini C. Capillary permeability in fibromyalgia. J Rheumatol. 1994;21:1328–31.
Lee JH, Cho KI, Kim SM, Lee HG, Kim TI. Arterial stiffness in female patients with fibromyalgia and its relationship to chronic emotional and physical stress. Korean Circ J. 2011;41:596–602.
Kim Y, Kim G-T, Kang J. Carotid arterial stiffness and cardiometabolic profiles in women with fibromyalgia. Biomedicines. 2021;9:1786.
Gurer G, Sendur OF, Ay C. Serum lipid profile in fibromyalgia women. Clin Rheumatol. 2006;25:300–3.
Vandooren J, Itoh Y. Alpha-2-macroglobulin in inflammation, immunity and infections. Front Immunol. 2021;12: 803244.
Baraniuk JN, Casado B, Maibach H, Clauw DJ, Pannell LK, Hess S. A chronic fatigue syndrome - related proteome in human cerebrospinal fluid. BMC Neurol. 2005;5:1–19.
Jensen PEH, Humle Jørgensen S, Datta P, Sørensen PS. Significantly increased fractions of transformed to total alpha2-macroglobulin concentrations in plasma from patients with multiple sclerosis. Biochim Biophys Acta. 2004;1690:203–7.
• De Castro JC, Wang D, Chien GCC. Regenerative medicine for neuropathic pain: physiology, ultrasound and therapies with a focus on alpha-2-macroglobulin. Pain Manag. 2022;12:779–93. This review indicates that A2M can be efficacious in reducing neuropathic pain due to its conformational change during activation and specificity of action on various cytokines.
de Castro JC, Wang D, Strakowski J, Emril DR, Chang Chien GC. Alpha-2 macroglobulin for the treatment of neuroma pain in the stump of a below-knee amputee patient. Pain Manag. 2023;13:335–41.
Thompson K, Klein D, Campbell K, Gonzlez-Lomas G, Alaia M, Strauss E, et al. The effectiveness of alpha-2-macroglobulin injections for osteoarthritis of the knee. Arthrosc J Arthrosc Relat Surg. 2021;37: e80.
Zhu M, Zhao B, Wei L, Wang S. alpha-2-macroglobulin, a native and powerful proteinase inhibitor, prevents cartilage degeneration disease by inhibiting majority of catabolic enzymes and cytokines. Curr Mol Biol Rep. 2021;7:1–7.
Arranz LI, Canela MÃ, Rafecas M. Dietary aspects in fibromyalgia patients: results of a survey on food awareness, allergies, and nutritional supplementation. Rheumatol Int. 2012;32:2615–21.
Berger K, Moeller MJ. Cofilin-1 in the podocyte: a molecular switch for actin dynamics. Int Urol Nephrol. 2011;43:273–5.
Hamilton G. Cyclophilin A as a target of Cisplatin chemosensitizers. Curr Cancer Drug Targets. 2014;14:46–58.
Samatov TR, Wicklein D, Tonevitsky AG. L1CAM: Cell adhesion and more. Prog Histochem Cytochem. 2016;51:25–32.
Ling M, Murali M. Analysis of the complement system in the clinical immunology laboratory. Clin Lab Med. 2019;39:579–90.
Fukazawa N, Yokoyama S, Eiraku M, Kengaku M, Maeda N. Receptor type protein tyrosine phosphatase zeta-pleiotrophin signaling controls endocytic trafficking of DNER that regulates neuritogenesis. Mol Cell Biol. 2008;28:4494–506.
Liu L, Guo H, Song A, Huang J, Zhang Y, Jin S, et al. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol. 2020;21:32.
Rani L, Minz RW, Arora A, Kannan M, Sharma A, Anand S, et al. Serum proteomic profiling in granumomatosis with polyangiitis using two-dimensional gel electrophoresis along with matrix assisted laser desorption ionization time of flight mass spectrometry. Int J Rheum Dis. 2014;17:910–9.
Lindberg I, Shu Z, Lam H, Helwig M, Yucer N, Laperle A, et al. The proSAAS chaperone provides neuroprotection and attenuates transsynaptic α-synuclein spread in rodent models of Parkinson’s disease. J Parkinsons Dis. 2022;12:1463–78.
Wajima T, Isbister GK, Duffull SB. A comprehensive model for the humoral coagulation network in humans. Clin Pharmacol Ther. 2009;86:290–8.
Li Z, He C, Liu Y, Wang D, Lin M, Liu C, et al. Association of Fetuin-B with subclinical atherosclerosis in obese Chinese adults. J Atheroscler Thromb. 2020;27:418–28.
Saigo K, Yoshida A, Sugano W, Ryo R. Yamaguchi N [Histidine-rich glycoprotein in blood during inflammation, surgical operation or hemodialysis]. Rinsho Ketsueki. 1990;31:1914–9.
Wu Z, Zhang Z, Lei Z, Lei P. CD14: biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019;48:24–31.
Piyaphanee N, Ma Q, Kremen O, Czech K, Greis K, Mitsnefes M, et al. Discovery and initial validation of α 1-B glycoprotein fragmentation as a differential urinary biomarker in pediatric steroid-resistant nephrotic syndrome. Proteomics- Clin Appl Clin Appl. 2011;5:334–42.
Lubbers R, Beaart-van de Voorde LJJ, van Leeuwen K, de Boer M, Gelderman KA, van den Berg MJ, et al. Complex medical history of a patient with a compound heterozygous mutation in C1QC. Lupus. 2019;28:1255–60.
Victor AR, Weigel C, Scoville SD, Chan WK, Chatman K, Nemer MM, et al. Epigenetic and posttranscriptional regulation of CD16 expression during human NK cell development. J Immunol. 2018;200:565–72.
Mukhopadhyay S, Heinz E, Porreca I, Alasoo K, Yeung A, Yang H-T, et al. Loss of IL-10 signaling in macrophages limits bacterial killing driven by prostaglandin E2. J Exp Med. 2020;217: e20180649.
Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5:181.
Chapman KR, Chorostowska-Wynimko J, Koczulla AR, Ferrarotti I, McElvaney NG. Alpha 1 antitrypsin to treat lung disease in alpha 1 antitrypsin deficiency: recent developments and clinical implications. Int J Chron Obstruct Pulmon Dis. 2018;13:419–32.
Cunden LS, Brophy MB, Rodriguez GE, Flaxman HA, Nolan EM. Biochemical and functional evaluation of the intramolecular disulfide bonds in the zinc-chelating antimicrobial protein human S100A7 (psoriasin). Biochemistry. 2017;56:5726–38.
Stanly TA, Fritzsche M, Banerji S, Shrestha D, Schneider F, Eggeling C, et al. The cortical actin network regulates avidity-dependent binding of hyaluronan by the lymphatic vessel endothelial receptor LYVE-1. J Biol Chem. 2020;295:5036–50.
Funding
Open access funding provided by HEAL-Link Greece. The publication of the article in OA mode was financially supported by HEAL-Link.
Author information
Authors and Affiliations
Contributions
Conceptualization: Dimitrios P. Bogdanos, Dimitrios G. Goulis, and Maria G. Grammatikopoulou; literature search and data analysis: Arriana Gkouvi and Sotirios G. Tsiogkas; writing—original draft preparation: Arriana Gkouvi; writing—review and editing: Helen Gika, Maria G. Grammatikopoulou, Dimitrios P. Bogdanos, and Dimitrios G. Goulis; supervision: Dimitrios G. Goullis and Maria G. Grammatikopoulou; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gkouvi, A., Tsiogkas, S.G., Bogdanos, D.P. et al. Proteomics in Patients with Fibromyalgia Syndrome: A Systematic Review of Observational Studies. Curr Pain Headache Rep (2024). https://doi.org/10.1007/s11916-024-01244-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11916-024-01244-4