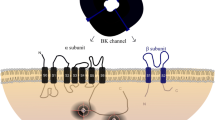
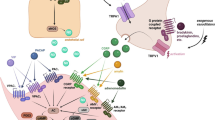
Abstract
Purpose of Review
Migraine remains a challenging condition to treat, thus highlighting the need for a better understanding of its molecular mechanisms. This review intends to unravel a new emerging target in migraine pathophysiology, the adenosine 5′-triphosphate-sensitive K+ (KATP) channel.
Recent Findings
KATP channel is a common denominator in the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) mediated intracellular cascades, both of which are involved in migraine. Intravenous infusion of KATP channel opener, levcromakalim, provoked migraine attack associated with dilation of extracerebral arteries in all persons with migraine.
Summary
Preclinical and clinical studies implicate KATP channels in migraine initiation. KATP channel is a novel therapeutic target for the acute and preventive treatment of migraine. Future studies are warranted to provide a better understanding of the role of KATP channel subgroups in migraine.


Similar content being viewed by others
Data Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Clemente Agostoni E, Barbanti P, Calabresi P, The Italian chronic migraine group, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20. https://doi.org/10.1186/s10194-019-1038-4.
Goadsby PJ, Holland PR. Migraine therapy: current approaches and new horizons. Neurotherapeutics. 2018;15:271–3.
Bohm PE, Stancampiano FF, Rozen TD. Migraine headache: updates and future developments. Mayo Clin Proc. 2018;93:1648–53.
Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394:1765–74.
Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17:174–82.
Ashina M, Hansen JM, BOÁ D, Olesen J. Human models of migraine-short-term pain for long-term gain. Nat Rev Neurol. 2017;13:713–24. https://doi.org/10.1038/nrneurol.2017.137.
Ashina M, Hansen JM, Olesen J. Pearls and pitfalls in human pharmacological models of migraine: 30 years’ experience. Cephalalgia. 2013;33:540–53.
Schytz HW, Schoonman GG, Ashina M. What have we learnt from triggering migraine? Curr Opin Neurol. 2010;23:259–65.
Al-Karagholi MAM, Hansen JM, Severinsen J, Jansen-Olesen I, Ashina M. The KATP channel in migraine pathophysiology: a novel therapeutic target for migraine. J Headache Pain. 2017;18:90. https://doi.org/10.1186/s10194-017-0800-8.
Guo S, Olesen J, Ashina M. Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase. Brain. 2014;137:2951–9.
Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–6.
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61.
Ashina M, Bendtsen L, Jensen R, Schifter S, Olesen J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86:133–8.
Schubert R, Serebryakov VN, Mewes H, Hopp HH. Iloprost dilates rat small arteries: role of K(ATP)- and K(Ca)-channel activation by cAMP-dependent protein kinase. Am J Physiol Heart Circ Physiol. 1997;272:H1147–56. https://doi.org/10.1152/ajpheart.1997.272.3.h1147.
Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Coy DHIsolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–74.
Jansen-Olesen I, Mortensen A, Edvinsson L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–6.
Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328.
Al-Karagholi MAM, Hansen JM, Severinsen J, Jansen-Olesen I, Ashina M. The KATP channel in migraine pathophysiology: a novel therapeutic target for migraine. J Headache Pain. 2017;18:1–9.
Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab. 1987;7:720–8.
Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–86.
Fahrenkrug J. PACAP-a multifacetted neuropeptide. Chronobiol Int. 2006;23(1-2):53-61. https://doi.org/10.1080/07420520500464569.
Tajti J, Uddman R, Möller S, Sundler F, Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst. 1999;76:176–83.
Syed AU, Koide M, Braas KM, May V, Wellman GC. Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: implications for migraine. J Mol Neurosci. 2012;48:574–83.
Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132:16–25.
Ghanizada H, Al-Karagholi MA-M, Arngrim N, Olesen J, Ashina M. PACAP27 induces migraine-like attacks in migraine patients. Cephalalgia. 2020;40(1):57–67. https://doi.org/10.1177/0333102419864507.
Zhang YZ, Sjőlund B, Moller K, Håkanson R, Sundler F. Pituitary adenylate cyclase activating peptide produces a marked and long-lasting depression of a C-fibre-evoked flexion reflex. Neuroscience. 1993;57:733–7.
Gulbenkian S, Uddman R, Edvinsson L. Neuronal messengers in the human cerebral circulation. Peptides. 2001;22:995–1007.
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357.
Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8.
Hosoya M, Onda H, Ogi K, Masuda Y, Miyamoto Y, Ohtaki T, et al. Molecular cloning and functional expression of rat cDNAs encoding the receptor for pituitary adenylate cyclase activating polypeptide (PACAP). Biochem Biophys Res Commun. 1993;194:133–43.
Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–36.
Amin FM, Hougaard A, Schytz HW, Asghar MS, Lundholm E, Parvaiz AI, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain. 2014;137:779–94.
Study to evaluate the efficacy and safety of AMG 301 in migraine prevention - Full Text View -ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03238781. Accessed 1 May 2020.
Pellesi L, Al-Karagholi MA-M, Chaudhry BA, Lopez CL, Snellman J, Hannibal J, et al. Two-hour infusion of vasoactive intestinal polypeptide induces delayed headache and extracranial vasodilation in healthy volunteers. Cephalalgia. 2020;40:1212–23. https://doi.org/10.1177/0333102420937655.
The effects of a long-lasting infusion of vasoactive intestinal peptide (VIP) in episodic migraine patients -ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04260035. Accessed 19 April 2020.
Pardutz A, Schoenen J. NSAIDs in the acute treatment of migraine: a review of clinical and experimental data. Pharmaceuticals. 2010;3:1966–87.
Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000.
Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia. 2012;32:822–33.
Wienecke T, Olesen J, Ashina M. Discrepancy between strong cephalic arterial dilatation and mild headache caused by prostaglandin D2 (PGD2). Cephalalgia. 2011;31:65–76.
Wienecke T, Olesen J, Ashina M. Prostaglandin I2 (epoprostenol) triggers migraine-like attacks in migraineurs. Cephalalgia. 2010;30:179–90.
Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66.
Liu Y, Shakur Y, Yoshitake M, Kambayashi JI. Cilostazol (Pletal®): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001;19:369–86.
Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42.
Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport. 1993;4:1027–30.
Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38:17–24.
Hansen JM, Pedersen DL, Larsen VA, Sánchez-Del-Rio M, Alvarez Linera JR, Olesen J, et al. Magnetic resonance angiography shows dilatation of the middle cerebral artery after infusion of glyceryl trinitrate in healthy volunteers. Cephalalgia. 2007;27:118–27.
Lassen LH, Thomsen LL, Olesen J. Histamine induces migraine via the H1-receptor. Support for the NO hypothesis of migraine. Neuroreport. 1995;6:1475–9.
Faraci FM, Brian JE. Nitric oxide and the cerebral circulation. Stroke. 1994;25:692–703.
Kruuse C, Thomsen LL, Birk S, Olesen J. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain. 2003;126:241–7.
Christensen CE, Younis S, Lindberg U, et al. Intradural artery dilation during experimentally induced migraine attacks. [published online ahead of print, 2020 Jul 21]. Pain. 2020. https://doi.org/10.1097/j.pain.0000000000002008.
Niehaus L, Gottschalk S, Weber U. Effect of drug-induced vasodilatation of basal brain arteries with nitroglycerin on blood flow velocity and volume flow in the middle cerebral artery. Ultraschall Med. 1998;19:225–9.
Armstead WM. Role of ATP-sensitive K+ channels in cGMP-mediated pial artery vasodilation. Am J Physiol Heart Circ Physiol. 1996;270:H423–6. https://doi.org/10.1152/ajpheart.1996.270.2.h423.
Hempelmann RG, Seebeck J, Kruse ML, Ziegler A, Mehdorn HM. Role of potassium channels in the relaxation induced by the nitric oxide (NO) donor DEA/NO in the isolated rat basilar artery. Neurosci Lett. 2001;313:21–4.
Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8.
Schwanstecher C, Panten U. Tolbutamide- and diazoxide-sensitive K+ channel in neurons of substantia nigra pars reticulata. Naunyn Schmiedeberg's Arch Pharmacol. 1993;348:113–7.
Ashford MLJ, Sturgess NC, Trout NJ, Gardner NJ, Hales CN. Adenosine-5′-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch - Eur J Physiol. 1988;412:297–304.
Bernardi H, De Weille JR, Epelbaum J, Mourre C, Amoroso S, Slama A, et al. ATP-modulated K+ channels sensitive to antidiabetic sulfonylureas are present in adenohypophysis and are involved in growth hormone release. Proc Natl Acad Sci U S A. 1993;90:1340–4.
Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;80–245:177–80.
Ashcroft FM, Harrison DE, Ashcroft SJH. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature. 1984;312:446–8.
Cook DL, Hales N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–3.
Chutkow WA, Makielski JC, Nelson DJ, Burant CF, Fan Z. Alternative splicing of sur2 exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channel. J Biol Chem. 1999;274:13656–65.
Aguilar-Bryan L, Clement JP IV, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of K(ATP) channels. Physiol Rev. 1998;78:227–45.
Kuang Q, Purhonen P, Hebert H. Structure of potassium channels. Cell Mol Life Sci. 2015;72:3677–93.
Rodrigo G, Standen N. ATP-sensitive potassium channels. Curr Pharm Des. 2005;11:1915–40.
Ploug KB, Amrutkar DV, Baun M, Ramachandran R, Iversen A, Lund TM, et al. ATP channel openers in the trigeminovascular system. Cephalalgia. 2012;32:55–65.
Inagaki N, Seino S. ATP-sensitive potassium channels: structures, functions, and pathophysiology. Jpn J Physiol. 1998;48:397–412.
Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–8.
Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–94.
Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–76.
Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–232.
Faraci FM, Heistad DD. Role of ATP-sensitive potassium channels in the basilar artery. Am J Physiol Heart Circ Physiol. 1993;264:H8–H13. https://doi.org/10.1152/ajpheart.1993.264.1.h8.
Nagao T, Sadoshima S, Kamouchi M, Fujishima M. Cromakalim dilates rat cerebral arteries in vitro. Stroke. 1991;22:221–4.
McPherson GA, Stork AP. The resistance of some rat cerebral arteries to the vasorelaxant effect of cromakalim and other K+ channel openers. Br J Pharmacol. 1992;105:51–8.
Masuzawa K, Asano M, Matsuda T, Imaizumi Watanabe YM. Possible involvement of ATP-sensitive K+ channels in the relaxant response of dog middle cerebral artery to cromakalim. J Pharmacol Exp Ther. 1990;255:818–25.
Iwamoto T, Nishimura N, Morita T, Sukamoto T. Differential vasorelaxant effects of K(+)-channel openers and Ca(2+)-channel blockers on canine isolated arteries. J Pharm Pharmacol. 1993;45:292–7.
Ksoll E, Parsons AA, Mackert JRL, Schilling L, Wahl M. Analysis of cromakalim-, pinacidil-, and nicorandil-induced relaxation of the 5-hydroxytryptamine precontracted rat isolated basilar artery. Naunyn Schmiedeberg's Arch Pharmacol. 1991;343:377–83.
Ploug KB, Sørensen MA, Strøbech L, Klaerke DA, Hay-Schmidt A, Sheykhzade M, et al. KATP channels in pig and human intracranial arteries. Eur J Pharmacol. 2008;601:43–9.
Jahangir A, Terzic A. KATP channel therapeutics at the bedside. J Mol Cell Cardiol. 2005;39:99–112.
Mannhold R. KATP channel openers: structure-activity relationships and therapeutic potential. Med Res Rev. 2004;24:213–66.
Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–91.
Roland E. Safety profile of an anti-anginal agent with potassium channel opening activity: an overview. Eur Heart J. 1993;14:48–52.
Thomas P, Dixon M, Winterton S, Sheridan D. Acute haemodynamic effects of cromakalim in patients with angina pectoris. Br J Clin Pharmacol. 1993;29:325–31.
Williams AJ, Lee TH, Vyse T, Chiew F, Cochrane GM, Williams AJ, et al. Attenuation of nocturnal asthma by cromakalim. Lancet. 1990;336:334–6.
Kidney JC, Fuller RW, Worsdell YM, Lavender EA, Chung KF, Barnes PJ. Effect of an oral potassium channel activator, BRL 38227, on airway function and responsiveness in asthmatic patients: comparison with oral salbutamol. Thorax. 1993;48:130–3.
Al-Karagholi MA, Ghanizada H, Hansen JM, Skovgaard LT, Olesen J, Larsson HBW, et al. Levcromakalim, an adenosine triphosphate-sensitive potassium channel opener, dilates extracerebral but not cerebral arteries. Headache. 2019;59:1468–80.
•• Al-Karagholi MAM, Hansen JM, Guo S, Olesen J, Ashina M. Opening of ATP-sensitive potassium channels causes migraine attacks: a new target for the treatment of migraine. Brain. 2019;142:2644–54. Important study supporting the importance of KATP channels in migraine.
Al-Karagholi MAM, Ghanizada H, Hansen JM, Aghazadeh S, Skovgaard LT, Olesen J, et al. Extracranial activation of ATP-sensitive potassium channels induces vasodilation without nociceptive effects. Cephalalgia. 2019;39:1789–97.
Barbanti P, Egeo G. Mitsikostas DDTrigeminal-targeted treatments in migraine: is 60% the magic number? Headache. 2019;59:1659–61.
Glibenclamide: a review. Drugs. 1971;1:116–40. https://doi.org/10.2165/00003495-197101020-00002.
•• Christensen SL, Munro G, Petersen S, Shabir A, Jansen-Olesen I, Kristensen DM, et al. ATP sensitive potassium (KATP) channel inhibition: a promising new drug target for migraine. Cephalalgia. 2020. https://doi.org/10.1177/0333102420925513. Study supporting a possible role for KATP channel blockers in migraine treatment.
•• Gozalov A, Jansen-Olesen I, Klaerke D, Olesen J. Role of KATP channels in cephalic vasodilatation induced by calcitonin gene-related peptide, nitric oxide, and transcranial electrical stimulation in the rat. Headache. 2008;48:1202–13. Important study supporting the importance of KATP channels in migraine.
Bruch L, Rubel S, Kästner A, Gellert K, Gollasch M, Witt C. Pituitary adenylate cyclase activating peptides relax human pulmonary arteries by opening of K(ATP) and K(CA) channels. Thorax. 1998;53:586–7.
MAM Al‐Karagholi H, Ghanizada L, Kokoti Paulsen JS, Hansen JM, Ashina M. Effect of K channel blocker glibenclamide on levcromakalim-induced headache. Cephalalgia 2020;40(10):1045–54.
Ashford MLJ, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch - Eur J Physiol. 1990;415:479–83.
Röper J, Ashcroft FM. Metabolic inhibition and low internal ATP activate K-ATP channels in rat dopaminergic substantia nigra neurones. Pflugers Arch - Eur J Physiol. 1995;430:44–54.
Ohno-Shosaku T, Yamamoto C. Identification of an ATP-sensitive K+ channel in rat cultured cortical neurons. Pflugers Arch. 1992;422:260–6.
Häusser MA, de Weille JR, Lazdunski M. Activation by cromakalim of pre- and post-synaptic ATP-sensitive K+ channels in substantia nigra. Biochem Biophys Res Commun. 1991;174:909–14.
Maneuf YP, Duty S, Hille CJ, Crossman AR, Brotchie JM. Modulation of GABA transmission by diazoxide and cromakalim in the globus pallidus: implications for the treatment of Parkinson’s disease. Exp Neurol. 1996;139:12–6.
Hoffmann J, Charles A. Glutamate and its receptors as therapeutic targets for migraine. Neurotherapeutics. 2018;15:361–70.
Yee AG, Lee SM, Hunter MR, Glass M, Freestone PS, Lipski J. Effects of the Parkinsonian toxin MPP+ on electrophysiological properties of nigral dopaminergic neurons. Neurotoxicology. 2014;45:1–11.
Kyle BD, Hurst S, Swayze RD, Sheng J, Braun AP. Specific phosphorylation sites underlie the stimulation of a large conductance, Ca2+−activated K+ channel by cGMP-dependent protein kinase. FASEB J. 2013;27:2027–38.
So YP, Jeong HL, Chi DK, Won SL, Won SP, Han J, et al. Cilostazol suppresses superoxide production and expression of adhesion molecules in human endothelial cells via mediation of cAMP-dependent protein kinase-mediated maxi-K channel activation. J Pharmacol Exp Ther. 2006;317:1238–45.
Wulf-Johansson H, Amrutkar DV, Hay-Schmidt A, Poulsen AN, Klaerke DA, Olesen J, et al. Localization of large conductance calcium-activated potassium channels and their effect on calcitonin gene-related peptide release in the rat trigemino-neuronal pathway. Neuroscience. 2010;167:1091–102.
Wang Y, Mathers DA. Ca(2+)-Dependent K+ channels of high conductance in smooth muscle cells isolated from rat cerebral arteries. J Physiol. 1993;462:529–45.
Al-Karagholi MAM, Gram C, Nielsen CAW, Ashina M. Targeting BKCa channels in migraine: rationale and perspectives. CNS Drugs. 2020;34:325–35.
MAM Al‐Karagholi H, Ghanizada L, Kokoti Paulsen JS, Hansen JM, Ashina M. Opening of BK channels alters cerebral hemodynamic and causes headache in healthy volunteers . Cephalalgia 2020;40(11):1145–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
MMK has acted as an invited speaker for Novartis and received travel grant from ElectroCore, LLC. MA is a consultant, speaker, or scientific advisor for Allergan, Amgen, Alder, ATI, Eli Lilly, Lundbeck, Novartis, and Teva; primary investigator for Alder, Amgen, Allergan, Eli Lilly, Novartis, and Teva trials. MA has no ownership interest and does not own stocks of any pharmaceutical company. MA serves as associate editor of Cephalalgia; associate editor of Headache; associate editor of the Journal of Headache and Pain. MA is President of the International Headache Society. LK declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethics Approval
Not applicable
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Episodic Migraine
Rights and permissions
About this article
Cite this article
Kokoti, L., Al-Karagholi, M.AM. & Ashina, M. Latest Insights into the Pathophysiology of Migraine: the ATP-Sensitive Potassium Channels. Curr Pain Headache Rep 24, 77 (2020). https://doi.org/10.1007/s11916-020-00911-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11916-020-00911-6