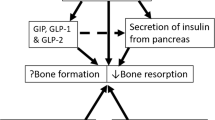
Abstract
Purpose of Review
To describe recent advances in the understanding of how gut-derived hormones regulate bone homeostasis in humans with emphasis on pathophysiological and therapeutic perspectives in diabetes.
Recent Findings
The gut-derived incretin hormone glucose-dependent insulinotropic polypeptide (GIP) is important for postprandial suppression of bone resorption. The other incretin hormone, glucagon-like peptide 1 (GLP-1), as well as the intestinotrophic glucagon-like peptide 2 (GLP-2) has been shown to suppress bone resorption in pharmacological concentrations, but the role of the endogenous hormones in bone homeostasis is uncertain. For ambiguous reasons, both patients with type 1 and type 2 diabetes have increased fracture risk. In diabetes, the suppressive effect of endogenous GIP on bone resorption seems preserved, while the effect of GLP-2 remains unexplored both pharmacologically and physiologically. GLP-1 receptor agonists, used for the treatment of type 2 diabetes and obesity, may reduce bone loss, but results are inconsistent.
Summary
GIP is an important physiological suppressor of postprandial bone resorption, while GLP-1 and GLP-2 may also exert bone-preserving effects when used pharmacologically. A better understanding of the actions of these gut hormones on bone homeostasis in patients with diabetes may lead to new strategies for the prevention and treatment of skeletal frailty related to diabetes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Crofford OB, Genuth S, Baker L. Diabetes control and complications trial (DCCT): results of feasibility study. Diabetes Care. 1987;10:1–19.
UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16.
Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–44.
Wang H, Ba Y, Xing Q, Du J-L. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9:e024067.
Dou J, Wang J, Zhang Q. Differences in the roles of types 1 and 2 diabetes in the susceptibility to the risk of fracture: a systematic review and meta-analysis. Diabetol Metab Syndr. 2021;13:84.
Bjarnason NH, Henriksen EEGG, Alexandersen P, Christgau S, Henriksen DB, Christiansen C. Mechanism of circadian variation in bone resorption. Bone. 2002;30:307–13.
Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30:886–90.
Hygum K, Starup-Linde J, Harsløf T, Jørgensen NR, Hartmann B, Holst JJ, Langdahl BL. The diurnal variation of bone formation is attenuated in adult patients with type 2 diabetes. Eur J Endocrinol 2019;181:221–231. This study compares bone turnover markers during 24 hours in patients with T1D, T2D and healthy subjects. Despite the small sample size, the study confirms the pronounced diurnal variability of CTX-I.
Holst JJ, Gasbjerg LS, Rosenkilde MM. The role of incretins on insulin function and glucose homeostasis. Endocrinology. 2021;162:bqab065.
Nauck MA, Quast DR, Wefers J, Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metab. 2021;23:5–29.
Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561–83.
Clowes JA, Allen HC, Prentis DM, Eastell R, Blumsohn A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab. 2003;88:4867–73.
Henriksen DB, Alexandersen P, Bjarnason NH, Vilsbøll T, Hartmann B, Henriksen EEG, Byrjalsen I, Krarup T, Holst JJ, Christiansen C. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res. 2003;18:2180–9.
Lund A, Bagger JI, Christensen M, Frost M, Jorgensen NR, Storkholm JH, Hansen CP, Holst JJ, Vilsboll T, Knop FK. [Abstract] Gut hormones, rather than glucose or insulin, are the main drivers of diminished bone resorption in the postabsorptive state. In: DIABETOLOGIA. Springer 233 Spring street, New York , NY 10013 USA; 2016. pp. S234–S235.
Mabilleau G, Gobron B, Bouvard B, Chappard D. Incretin-based therapy for the treatment of bone fragility in diabetes mellitus. Peptides. 2018;100:108–13.
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab. 2020;46:101102.
Wallis K, Walters JRF, Gabe S. Short bowel syndrome: the role of GLP-2 on improving outcome. Curr Opin Clin Nutr Metab Care. 2009;12:526–32.
Szulc P. Bone turnover: Biology and assessment tools. Best Pract Res Clin Endocrinol Metab. 2018;32:725–38.
Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol. 2008;61:577–87.
Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 2017;28:2541–2556.
Westberg-Rasmussen S, Starup-Linde J, Hermansen K, Holst JJ, Hartmann B, Vestergaard P, Gregersen S. Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone. 2017;97:261–6.
Stensen S, Gasbjerg LS, Helsted MM, Hartmann B, Christensen MB, Knop FK. GIP and the gut-bone axis – physiological, pathophysiological and potential therapeutic implications. Peptides. 2019. https://doi.org/10.1016/j.peptides.2019.170197.
Maagensen H, Junker AE, Jørgensen NR, Gluud LL, Knop FK, Vilsbøll T. Bone turnover markers in patients with nonalcoholic fatty liver disease and/or type 2 diabetes during oral glucose and isoglycemic intravenous glucose. J Clin Endocrinol Metab. 2018;103:2042–9.
Elrich H, Stimmler L, Hlad CJ, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–82.
Gasbjerg LS, Bergmann NC, Stensen S, Christensen MB, Rosenkilde MM, Holst JJ, Nauck M, Knop FK. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides. 2020. https://doi.org/10.1016/j.peptides.2019.170183.
Gasbjerg LS, Helsted MM, Hartmann B, Jensen MH, Gabe MBN, Sparre-Ulrich AH, Veedfald S, Stensen S, Lanng AR, Bergmann NC, Christensen MB, Vilsbøll T, Holst JJ, Rosenkilde MM, Knop FK. Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes. 2019;68:906–17.
Basu R, Peterson J, Rizza R, Khosla S. Effects of physiological variations in circulating insulin levels on bone turnover in humans. J Clin Endocrinol Metab. 2011;96:1450–5.
Ivaska KK, Heliövaara MK, Ebeling P, Bucci M, Huovinen V, Kalervo Väänänen H, Nuutila P, Koistinen HA. The effects of acute hyperinsulinemia on bone metabolism. Endocr Connect. 2015;4:155–62.
Helsted MM, Gasbjerg LS, Lanng AR, Bergmann NC, Stensen S, Hartmann B, Christensen MB, Holst JJ, Vilsbøll T, Rosenkilde MM, Knop FK. The role of endogenous GIP and GLP-1 in postprandial bone homeostasis. Bone. 2020. https://doi.org/10.1016/j.bone.2020.115553.
Christensen MB, Lund A, Calanna S, Jørgensen NR, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J Clin Endocrinol Metab 2018;103:288–294. This study supports a preserved effect of GIP on bone resorption in T1D. Furthermore, it supports that GIP acts on bone cells directly rather than through GIP-induced insulin secretion.
Dupre J, Ross SA, Watson D, Brown JC (1973) Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 37:826–828.
Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801.
Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–9.
Campbell JE. Targeting the GIPR for obesity: To agonize or antagonize? Potential mechanisms. Mol Metab. 2020;46:101139.
Bergmann NC, Lund A, Gasbjerg LS, Meessen ECE, Andersen MM, Bergmann S, Hartmann B, Holst JJ, Jessen L, Christensen MB, Vilsbøll T, Knop FK. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia. 2019;62:665–75.
Bergmann NC, Gasbjerg LS, Heimbürger SM, Krogh LSL, dela F, Hartmann B, Holst JJ, Jessen L, Christensen MB, Vilsbøll T, Lund A, Knop FK. No acute effects of exogenous glucose-dependent insulinotropic polypeptide on energy intake, appetite, or energy expenditure when added to treatment with a long-acting glucagon-like peptide 1 receptor agonist in men with type 2 diabetes. Diabetes Care. 2020;43:588–96.
Asmar M, Simonsen L, Madsbad S, Stallknecht B, Holst JJ, Bülow J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes. 2010;59:2160–3.
Asmar M, Simonsen L, Asmar A, Holst JJ, Dela F, Bülow J. Insulin plays a permissive role for the vasoactive effect of gip regulating adipose tissue metabolism in humans. J Clin Endocrinol Metab. 2016;101:3155–62.
Asmar M, Asmar A, Simonsen L, Dela F, Holst JJ, Bülow J. GIP-induced vasodilation in human adipose tissue involves capillary recruitment. Endocr Connect. 2019;8:806–13.
Gasbjerg LS, Helsted MM, Stensen S, et al. Endogenous glucose-dependent insulinotropic polypeptide (GIP) facilitates postprandial intestinal lipid uptake. Diabetologia. 2022;65:S260.
Bollag RJ, Zhong Q, Ding KH, Phillips P, Zhong L, Qin F, Cranford J, Mulloy AL, Cameron R, Isales CM. Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol Cell Endocrinol. 2001;177:35–41.
Skov-Jeppesen K, Svane MS, Martinussen C, Gabe MBN, Gasbjerg LS, Veedfald S, Bojsen-Møller KN, Madsbad S, Holst JJ, Rosenkilde MM, Hartmann B. GLP-2 and GIP exert separate effects on bone turnover: a randomized, placebo-controlled, crossover study in healthy young men. Bone. 2019;125:178–85.
Skov-Jeppesen K, Hepp N, Oeke J, Hansen MS, Jafari A, Svane MS, Balenga N, Olson JA Jr, Frost M, Kassem M, Madsbad S, Beck Jensen JE, Holst JJ, Rosenkilde MM, Hartmann B. The antiresorptive effect of GIP, but not GLP-2, is preserved in patients with hypoparathyroidism-a randomized crossover study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2021;36:1448–58.
Nissen A, Christensen M, Knop FK, Vilsbøll T, Holst JJ, Hartmann B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab. 2014;99:E2325–9.
Gabe MBN, Skov-Jeppesen K, Gasbjerg LS, Schiellerup SP, Martinussen C, Gadgaard S, Boer GA, Oeke J, Torz LJ, Veedfald S, Svane MS, Bojsen-Møller KN, Madsbad S, Holst JJ, Hartmann B, Rosenkilde MM. GIP and GLP-2 together improve bone turnover in humans supporting GIPR-GLP-2R co-agonists as future osteoporosis treatment. Pharmacol Res. 2022;176:106058.
Bergmann NC, Lund A, Gasbjerg LS, Jørgensen NR, Jessen L, Hartmann B, Holst JJ, Christensen MB, Vilsbøll T, Knop FK. Separate and combined effects of GIP and GLP-1 infusions on bone metabolism in overweight men without diabetes. J Clin Endocrinol Metab. 2019. https://doi.org/10.1210/jc.2019-00008.
Bollag RJ, Zhong Q, Phillips P, Min L, Zhong L, Cameron R, Mulloy AL, Rasmussen H, Qin F, Ding KH, Isales CM. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors 1. Endocrinology. 2000;141:1228–35.
Torekov SS, Harsløf T, Rejnmark L, Eiken P, Jensen JB, Herman AP, Hansen T, Pedersen O, Holst JJ, Langdahl BL. A functional amino acid substitution in the glucose-dependent insulinotropic polypeptide receptor (GIPR) gene is associated with lower bone mineral density and increased fracture risk. J Clin Endocrinol Metab. 2014;99:E729–33.
Kizilkaya HS, Sørensen KV, Kibsgaard CJ, Gasbjerg LS, Hauser AS, Sparre-Ulrich AH, Grarup N, Rosenkilde MM. Loss of function glucose-dependent insulinotropic polypeptide receptor variants are associated with alterations in bmi, bone strength and cardiovascular outcomes. Front Cell Dev Biol. 2021;9:749607.
Gasbjerg LS, Hartmann B, Christensen MB, Lanng AR, Vilsbøll T, Jørgensen NR, Holst JJ, Rosenkilde MM, Knop FK. GIP’s effect on bone metabolism is reduced by the selective GIP receptor antagonist GIP(3–30)NH2. Bone. 2020;130:115079.
Jorsal T, Rhee NA, Pedersen J, Wahlgren CD, Mortensen B, Jepsen SL, Jelsing J, Dalbøge LS, Vilmann P, Hassan H, Hendel JW, Poulsen SS, Holst JJ, Vilsbøll T, Knop FK. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018;61:284–94.
Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol Metab. 2020;46:101090.
Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jørgensen NR, Jensen J-EB, Holst JJ, Madsbad S, Torekov SS. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. 2015;100:2909–17.
Daniilopoulou I, Vlachou E, Lambrou GI, Ntikoudi A, Dokoutsidou E, Fasoi G, Govina O, Kavga A, Tsartsalis AN. The impact of GLP1 agonists on bone metabolism: a systematic review. Medicina (Mex). 2022. https://doi.org/10.3390/medicina58020224.
Nissen A, Marstrand S, Skov-Jeppesen K, Bremholm L, Hornum M, Andersen UB, Holst JJ, Rosenkilde MM, Hartmann B. A pilot study showing acute inhibitory effect of GLP-1 on the bone resorption marker CTX in humans. JBMR Plus. 2019;3:e10209.
Gasbjerg LS, Bari EJ, Christensen M, Knop FK. Exendin(9-39)NH2 : Recommendations for clinical use based on a systematic literature review. Diabetes Obes Metab. 2021;23:2419–36.
Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001;142:521–7.
Drucker DJ. The discovery of GLP-2 and Development of teduglutide for short bowel syndrome. ACS Pharmacol Transl Sci. 2019;2:134–42.
Haderslev KV, Jeppesen PB, Hartmann B, Thulesen J, Sorensen HA, Graff J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Staun M, Mortensen PB. Short-term administration of glucagon-like peptide-2. Effects on bone mineral density and markers of bone turnover in short-bowel patients with no colon. Scand J Gastroenterol. 2002;37:392–8.
Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, Holst JJ, Christiansen C. Disassociation of bone resorption and formation by GLP-2: a 14-day study in healthy postmenopausal women. Bone. 2007;40:723–9.
Gottschalck IB, Jeppesen PB, Hartmann B, Holst JJ, Henriksen DB. Effects of treatment with glucagon-like peptide-2 on bone resorption in colectomized patients with distal ileostomy or jejunostomy and short-bowel syndrome. Scand J Gastroenterol. 2008;43:1304–10.
Gottschalck IB, Jeppesen PB, Holst JJ, Henriksen DB. Reduction in bone resorption by exogenous glucagon-like peptide-2 administration requires an intact gastrointestinal tract. Scand J Gastroenterol. 2008;43:929–37.
Askov-Hansen C, Jeppesen PB, Lund P, Hartmann B, Holst JJ, Henriksen DB. Effect of glucagon-like peptide-2 exposure on bone resorption: Effectiveness of high concentration versus prolonged exposure. Regul Pept. 2013;181:4–8.
Skov-Jeppesen K, Veedfald S, Madsbad S, Holst JJ, Rosenkilde MM, Hartmann B. Subcutaneous GIP and GLP-2 inhibit nightly bone resorption in postmenopausal women: A preliminary study. Bone. 2021;152:116065.
Henriksen DB, Alexandersen P, Byrjalsen I, Hartmann B, Bone HG, Christiansen C, Holst JJ. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone. 2004;34:140–7.
Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, Holst JJ, Christiansen C. Four-month treatment with GLP-2 significantly increases hip BMD: A randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone. 2009;45:833–42.
Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in Type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52.
Vilsbøll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, Hansen T, Pedersen O, Holst JJ. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide - regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–903.
Christensen MB, Lund AB, Jørgensen NR, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide (GIP) reduces bone resorption in patients with type 2 diabetes. J Endocr Soc 2020;4:bvaa097. This study supports a (at least partly) preserved effect of GIP on bone resorption in T2D using infusion of GIP.
Stensen S, Gasbjerg LS, Krogh LL, et al. Effects of endogenous GIP in patients with type 2 diabetes. Eur J Endocrinol 2021;185:33–45. Using a novel GIP receptor antagonist, this study shows that endogenous GIP plays a role in the postprandial suppression of bone resorption and, also, that the effect of GIP on bone resorption in T2D is (at least partly) preserved.
Heimbürger SMN, Hoe B, Nielsen CN, Bergmann NC, Hartmann B, Holst JJ, Vilsbøll T, Dejgaard TF, Christensen MB, Knop FK. The effect of 6-day subcutaneous glucose-dependent insulinotropic polypeptide infusion on time in glycaemic range in patients with type 1 diabetes: a randomised, double-blind, placebo-controlled crossover trial. Diabetologia. 2021;64:2425–31.
Heimbürger SMN, Hoe B, Nielsen CN, et al. GIP affects hepatic fat and brown adipose tissue thermogenesis, but not white adipose tissue transcriptome in T1D. J Clin Endocrinol Metab. 2022;dgac542.
Lund A, Vilsboll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;300:1038–46.
Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. MECHANISMS IN ENDOCRINOLOGY: diabetes mellitus, a state of low bone turnover – a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137–57.
Liu XX, Jiang L, Liu Q, Zhang J, Niu W, Liu J, Zhang Q. Low bone turnover markers in young and middle-aged male patients with type 2 diabetes mellitus. J Diabetes Res. 2020;2020:6191468–8.
Starup-Linde J, Lykkeboe S, Handberg A, Vestergaard P, Høyem P, Fleischer J, Hansen TK, Poulsen PL, Laugesen E. Glucose variability and low bone turnover in people with type 2 diabetes. Bone. 2021;153:116159.
US Food and Drug Administration, Drug Approval Package: MOUNJARO. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/215866Orig1s000TOC.cfm. Accessed 12 Aug 2022.
Kong Q, Ruan Q, Fan C, Liu B-L, Reng L-P, Xu W. Evaluation of the risk of fracture in type 2 diabetes mellitus patients with incretins: an updated meta-analysis. Endokrynol Pol. 2021;72:319–28.
Cheng L, Hu Y, Li Y-Y, Cao X, Bai N, Lu T-T, Li G-Q, Li N, Wang A-N, Mao X-M. Glucagon-like peptide-1 receptor agonists and risk of bone fracture in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2019;35:e3168.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray J, Probstfield JL, Riddle MC, Solomon SD, Tardif JC, ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Zhang Y-S, Zheng Y-D, Yuan Y, Chen S-C, Xie B-C. Effects of anti-diabetic drugs on fracture risk: a systematic review and network meta-analysis. Front Endocrinol. 2021. https://doi.org/10.3389/fendo.2021.735824.
Hidayat K, Du X, Shi B-M. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in real-world use: systematic review and meta-analysis of observational studies. Osteoporos Int. 2019;30:1923–40.
Cai T-T, Li H-Q, Jiang L-L, Wang H-Y, Luo M-H, Su X-F, Ma J-H. Effects of GLP-1 receptor agonists on bone mineral density in patients with type 2 diabetes mellitus: a 52-week clinical study. BioMed Res Int. 2021;2021:3361309.
Hygum K, Harsløf T, Jørgensen NR, Rungby J, Pedersen SB, Langdahl BL. Bone resorption is unchanged by liraglutide in type 2 diabetes patients: a randomised controlled trial. Bone 2020;132:115197. The reported trial investigates the effects of a GLP-1RA, liraglutide, on bone metabolism as primary objective. After 26 weeks, liraglutide preserved BMD compared with placebo, while no effect was observed on CTX-I.
Mabilleau G. Use of GLP-1 mimetic in type 2 diabetes mellitus: is it the end of fragility fractures? Endocrine. 2015;48:1–2.
Hygum K, Harsløf T, Langdahl B, Starup-Linde J. Glucagon-like peptide-1 receptor agonists and fracture risk—limitations to current knowledge. Osteoporos Int. 2019;30:1709–10.
Johansen NJ, Dejgaard TF, Lund A, Schlüntz C, Hartmann B, Holst JJ, Vilsbøll T, Andersen HU, Knop FK. Effects of short-acting exenatide added three times daily to insulin therapy on bone metabolism in type 1 diabetes. Diabetes Obes Metab. 2022;24:221–7.
Dejgaard TF, Frandsen CS, Johansen NJ, Jorgensen NR, Knop FK, Madsbad S, Andersen HU. [Conference abstract] Liraglutide-Induced Weight Loss Does Not Compromise Bone Mineral Density or Markers of Bone Turnover in Overweight Patients with Type 1 Diabetes. The 77th Scientific Session of the ADA, San Diego, CA, USA. Diabetes. 2017;66:A305.
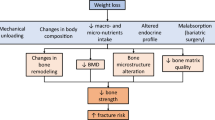
Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, Nguyen TV, Sainsbury A. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res. 2015;30:2168–78.
Harper C, Pattinson AL, Fernando HA, Zibellini J, Seimon RV, Sainsbury A. Effects of obesity treatments on bone mineral density, bone turnover and fracture risk in adults with overweight or obesity. Horm Mol Biol Clin Investig. 2016;28:133–49.
Leslie WD, Morin SN, Lix LM, Majumdar SR. Does diabetes modify the effect of FRAX risk factors for predicting major osteoporotic and hip fracture? Osteoporos Int. 2014;25:2817–24.
Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A, Womack CR, Palermo L, Black DM, Study of Osteoporotic Fractures (SOF) Research Group., Osteoporotic Fractures in Men (MrOS) Research Group., Health, Aging, and Body Composition (Health ABC) Research Group. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–92.
Khosla S, Samakkarnthai P, Monroe DG, Farr JN. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:685–97.
Naimi RM, Hvistendahl M, Enevoldsen LH, Madsen JL, Fuglsang S, Poulsen SS, Kissow H, Pedersen J, Nerup N, Ambrus R, Achiam MP, Svendsen LB, Holst JJ, Hartmann B, Hansen SH, Dragsted LO, Steensberg A, Mouritzen U, Hansen MB, Jeppesen PB. Glepaglutide, a novel long-acting glucagon-like peptide-2 analogue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2019;4:354–63.
Chailurkit L, Chanprasertyothin S, Rajatanavin R, Ongphiphadhanakul B. Reduced attenuation of bone resorption after oral glucose in type 2 diabetes. Clin Endocrinol (Oxf). 2008;68:858–62.
Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–55.
Karagiannis T, Avgerinos I, Liakos A, Del Prato S, Matthews DR, Tsapas A, Bekiari E. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022. https://doi.org/10.1007/s00125-022-05715-4.
Jensen NW, Clemmensen KKB, Jensen MM, Pedersen H, Færch K, Diaz LJ, Quist JS, Størling J. Associations between Postprandial Gut Hormones and Markers of Bone Remodeling. Nutrients. 2021;13:3197.
Leitch VD, Brassill MJ, Rahman S, Butterfield NC, Ma P, Logan JG, Boyde A, Evans H, Croucher PI, Batterham RL, Williams GR, Bassett JHD. PYY is a negative regulator of bone mass and strength. Bone. 2019;127:427–35.
Lafferty RA, Flatt PR, Irwin N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides. 2018;100:269–74.
Naot D, Musson DS, Cornish J. The activity of peptides of the calcitonin family in bone. Physiol Rev. 2019;99:781–805.
Villa I, Rubinacci A, Ravasi F, Ferrara AF, Guidobono F. Effects of Amylin on human osteoblast-like cells. Peptides. 1997;18:537–40.
Author information
Authors and Affiliations
Contributions
H.M. and M.H. searched and analyzed the relevant literature and drafted the manuscript. LSG prepared the figure. All authors wrote, edited, and approved the present manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
H.M. and M.M.H declare no conflict of interest. L.S.G. is minority shareholder of Antag Therapeutics. T.V. has served on scientific advisory panels, been part of speaker’s bureaus for, served as a consultant to and/or received research support from Abbot, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, GSK, MSD/Merck, Novo Nordisk, Sanofi, and Sun Pharmaceuticals. F.K.K. has served on scientific advisory panels, been part of speaker’s bureaus for, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Norgine, Novo Nordisk, Sanofi, ShouTi, SNIPR Biome, Zealand Pharma and Zucara; and is a minority shareholder of Antag Therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain original data from studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Bone and Diabetes
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maagensen, H., Helsted, M.M., Gasbjerg, L.S. et al. The Gut-Bone Axis in Diabetes. Curr Osteoporos Rep 21, 21–31 (2023). https://doi.org/10.1007/s11914-022-00767-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00767-2