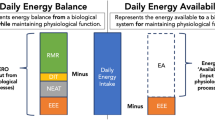
Abstract
Purpose of Review
Obesity is highly prevalent and is associated with bone fragility and fracture. The changing nutrient availability to bone in obesity is an important facet of bone health. The goal of this article is to summarize current knowledge on the effects of carbohydrate and dietary fat availability on bone, particularly in the context of other tissues.
Recent Findings
The skeleton is a primary site for fatty acid and glucose uptake. The trafficking of carbohydrates and fats into tissues changes with weight loss and periods of weight gain. Exercise acutely influences nutrient uptake into bone and may affect nutrient partitioning to bone. Bone cells secrete hormones that signal to the brain and other tissues information about its energetic state, which may alter whole-body nutrient trafficking.
Summary
There is a critical need for studies to address the changes that metabolic perturbations have on nutrient availability in bone.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ahmadi N, Sadr SM, Mohammadi MR, Mirzaei M, Mehrparvar AH, Yassini Ardekani SM, Sarebanhassanabadi M, Nilforoshan N, Mostafavi SA. Prevalence of abdominal obesity and metabolic syndrome in children and adolescents: a community based cross-sectional study. Iran J Public Health. 2020;49(2):360–8.
Liu AW, Song SO, Hayashi T, Sato KK, Kahn SE, Leonetti DL, Fujimoto WY, Boyko EJ. Change in CT-measured abdominal subcutaneous and visceral but not thigh fat areas predict future insulin sensitivity. Diabetes Res Clin Pract. 2019;154:17–26.
Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101(5):991–9.
Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res. 2013;471(4):1199–207.
Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567–73.
Rodriguez-Gomez I, Manas A, Losa-Reyna J, Rodriguez-Manas L, Chastin SFM, Alegre LM, et al. Associations between sedentary time, physical activity and bone health among older people using compositional data analysis. PLoS ONE. 2018;13(10):e0206013.
Tamme R, Jurimae J, Maestu E, Remmel L, Purge P, Mengel E, et al. Physical activity in puberty is associated with total body and femoral neck bone mineral characteristics in males at 18 years of age. Medicina (Kaunas). 2019;55(5).
Kim HY, Jung HW, Hong H, Kim JH, Shin CH, Yang SW, Lee YA. The role of overweight and obesity on bone health in Korean adolescents with a focus on lean and fat mass. J Korean Med Sci. 2017;32(10):1633–41.
Zeng FF, Wu BH, Fan F, Xie HL, Xue WQ, Zhu HL, Chen YM. Dietary patterns and the risk of hip fractures in elderly Chinese: a matched case-control study. J Clin Endocrinol Metab. 2013;98(6):2347–55.
Wolfel EM, Jahn-Rickert K, Schmidt FN, Wulff B, Mushumba H, Sroga GE, et al. Individuals with type 2 diabetes mellitus show dimorphic and heterogeneous patterns of loss in femoral bone quality. Bone. 2020;140:115556.
• Merlo K, Aaronson J, Vaidya R, Rezaee T, Chalivendra V, Karim L. In vitro-induced high sugar environments deteriorate human cortical bone elastic modulus and fracture toughness. J Orthop Res. 2020;38(5):972-83. This paper observed reductions in elastic modulus and fracture toughness in human cortical bone specimens incubated with ribose compared to bone samples that were not treated.
Yang L, Liu J, Shan Q, Geng G, Shao P. High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem Biophys Res Commun. 2020;522(2):471–8.
Pahwa H, Khan MT, Sharan K. Hyperglycemia impairs osteoblast cell migration and chemotaxis due to a decrease in mitochondrial biogenesis. Mol Cell Biochem. 2020;469(1-2):109–18.
Inzana JA, Kung M, Shu L, Hamada D, Xing LP, Zuscik MJ, Awad HA, Mooney RA. Immature mice are more susceptible to the detrimental effects of high fat diet on cancellous bone in the distal femur. Bone. 2013;57(1):174–83.
Wee NKY, Enriquez RF, Nguyen AD, Horsnell H, Kulkarni R, Khor EC, Herzog H, Baldock PA. Diet-induced obesity suppresses cortical bone accrual by a neuropeptide Y-dependent mechanism. Int J Obes (Lond). 2018;42(11):1925–38.
Sherk VD, Heveran CM, Foright RM, Johnson GC, Presby DM, Ferguson VL, MacLean PS. Sex differences in the effect of diet, obesity, and exercise on bone quality and fracture toughness. Bone. 2021;145:115840.
Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PCN, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43(2):230–7.
Picke AK, Sylow L, Moller LLV, Kjobsted R, Schmidt FN, Steejn MW, et al. Differential effects of high-fat diet and exercise training on bone and energy metabolism. Bone. 2018;116:120–34.
• Al Saedi A, Bermeo S, Plotkin L, Myers DE, Duque G. Mechanisms of palmitate-induced lipotoxicity in osteocytes. Bone. 2019;127:353-9. This paper observed decreases in RANKL, DKK1, and sclerostin expression in MLO-Y4 cells treated with palmitic acid compared to cells not treated. These conditions replicate what cells may experience in an environment exposed to an excess high-fat diet.
Al Saedi A, Myers DE, Stupka N, Duque G. 1,25(OH)2D3 ameliorates palmitate-induced lipotoxicity in human primary osteoblasts leading to improved viability and function. Bone. 2020;141:115672.
McCabe LR, Irwin R, Tekalur A, Evans C, Schepper JD, Parameswaran N, et al. Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone. 2019;118:20–31.
Mizokami A, Kawakubo-Yasukochi T, Hirata M. Osteocalcin and its endocrine functions. Biochem Pharmacol. 2017;132:1–8.
Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, Lanzano P, Deng L, Leibel RL, Rubin M, Nickolas T, Chung W, Zeltser LM, Williams KW, Pessin JE, Kousteni S. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543(7645):385–90.
•• Mosialou I, Shikhel S, Luo N, Petropoulou PI, Panitsas K, Bisikirska B, et al. Lipocalin -2 counteracts metabolic dysregulation in obesity and diabetes. J Exp Med. 2020;217(10). Bone is emerging as an endocrine organ and this paper demonstrates how reducing lipocalin-2 expression in mice leads to metabolic dysfunction, increases in fat mass, and glucose intolerance.
Bartelt A, Koehne T, Todter K, Reimer R, Muller B, Behler-Janbeck F, et al. Quantification of bone fatty acid metabolism and its regulation by adipocyte lipoprotein lipase. Int J Mol Sci. 2017;18(6).
Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105(13):5266–70.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69.
Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, da H, Aja S, Noh HL, Kim JK, Hussain MA, Thorek DLJ, Wolfgang MJ, Riddle RC. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017;114(52):E11238–E47.
Nguyen HH, Wu F, Oddy WH, Wills K, Winzenberg T, Jones G. Associations between dietary patterns and osteoporosis-related outcomes in older adults: a longitudinal study. Eur J Clin Nutr. 2021;75(5):792–800.
Qiu R, Cao WT, Tian HY, He J, Chen GD, Chen YM. Greater intake of fruit and vegetables is associated with greater bone mineral density and lower osteoporosis risk in middle-aged and elderly adults. PLoS ONE. 2017;12(1):e0168906.
Regu GM, Kim H, Kim YJ, Paek JE, Lee G, Chang N, et al. Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30-75 years using data from the Fourth and Fifth Korean National Health and Nutrition Examination Surveys (2008-2011). Nutrients. 2017;9(9).
Silva TRD, Martins CC, Ferreira LL, Spritzer PM. Mediterranean diet is associated with bone mineral density and muscle mass in postmenopausal women. Climacteric. 2019;22(2):162–8.
Perez-Rey J, Roncero-Martin R, Rico-Martin S, Rey-Sanchez P, Pedrera-Zamorano JD, Pedrera-Canal M, et al. Adherence to a Mediterranean diet and bone mineral density in Spanish premenopausal women. Nutrients. 2019;11(3).
Malmir H, Saneei P, Larijani B, Esmaillzadeh A. Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2018;57(6):2147–60.
Mu M, Wang SF, Sheng J, Zhao Y, Wang GX, Liu KY, Hu CL, Tao FB, Wang HL. Dietary patterns are associated with body mass index and bone mineral density in Chinese freshmen. J Am Coll Nutr. 2014;33(2):120–8.
Minematsu A, Nishii Y, Sakata S. High-fat/high-sucrose diet results in higher bone mass in aged rats. Bone Rep. 2018;8:18–24.
Tian L, Wang C, Xie Y, Wan S, Zhang K, Yu X. High fructose and high fat exert different effects on changes in trabecular bone micro-structure. J Nutr Health Aging. 2018;22(3):361–70.
Hafner H, Chang E, Carlson Z, Zhu A, Varghese M, Clemente J, et al. Lactational high-fat diet exposure programs metabolic inflammation and bone marrow adiposity in male offspring. Nutrients. 2019;11(6).
Zhang Z, Lin T, Meng Y, Hu M, Shu L, Jiang H, Gao R, Ma J, Wang C, Zhou X. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism. 2021;119:154767.
Sherk VD, Jackman MR, Higgins JA, Giles ED, Foright RM, Presby DM, et al. Impact of exercise and activity on weight regain and musculoskeletal health post-ovariectomy. Med Sci Sports Exerc. 2019;51(12):2465–73.
Li Z, Frey JL, Wong GW, Faugere MC, Wolfgang MJ, Kim JK, Riddle RC, Clemens TL. Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology. 2016;157(11):4094–103.
• Rendina-Ruedy E, Guntur AR, Rosen CJ. Intracellular lipid droplets support osteoblast function. Adipocyte. 2017;6(3):250-8. This paper provides evidence lipid uptake is required for osteoblasts in order to function properly.
Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24(2):e6–10.
Oh SR, Sul OJ, Kim YY, Kim HJ, Yu R, Suh JH, Choi HS. Saturated fatty acids enhance osteoclast survival. J Lipid Res. 2010;51(5):892–9.
Zoch ML, Abou DS, Clemens TL, Thorek DL, Riddle RC. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 2016;4:16004.
• Heinonen I, Kemppainen J, Fujimoto T, Knuuti J, Kalliokoski KK. Increase of glucose uptake in human bone marrow with increasing exercise intensity. Int J Sport Nutr Exerc Metab. 2019;29(3):254-8. Bone marrow adipose is often thought of only as an ectopic fat reservoir. This paper observed an increase in bone marrow glucose uptake in males exposed to moderate-intensity exercise compared to low-intensity exercise. This refutes the reservoir idea and shows a change in nutrient intake under increased energy demands.
Heinonen I, Kemppainen J, Kaskinoro K, Langberg H, Knuuti J, Boushel R, Kjaer M, Kalliokoski KK. Bone blood flow and metabolism in humans: effect of muscular exercise and other physiological perturbations. J Bone Miner Res. 2013;28(5):1068–74.
Rojas JM, Bolze F, Thorup I, Nowak J, Dalsgaard CM, Skydsgaard M, Berthelsen LO, Keane KA, Søeborg H, Sjögren I, Jensen JT, Fels JJ, Offenberg HK, Andersen LW, Dalgaard M. The effect of diet-induced obesity on toxicological parameters in the polygenic Sprague-Dawley rat model. Toxicol Pathol. 2018;46(7):777–98.
Montalvany-Antonucci CC, Zicker MC, Ferreira AVM, Macari S, Ramos-Junior ES, Gomez RS, Pereira TSF, Madeira MFM, Fukada SY, Andrade I Jr, Silva TA. High-fat diet disrupts bone remodeling by inducing local and systemic alterations. J Nutr Biochem. 2018;59:93–103.
Montalvany-Antonucci CC, Duffles LF, de Arruda JAA, Zicker MC, de Oliveira S, Macari S, Garlet GP, Madeira MFM, Fukada SY, Andrade I Jr, Teixeira MM, Mackay C, Vieira AT, Vinolo MA, Silva TA. Short-chain fatty acids and FFAR2 as suppressors of bone resorption. Bone. 2019;125:112–21.
Silva MJ, Eekhoff JD, Patel T, Kenney-Hunt JP, Brodt MD, Steger-May K, Scheller EL, Cheverud JM. Effects of high-fat diet and body mass on bone morphology and mechanical properties in 1100 advanced intercross mice. J Bone Miner Res. 2019;34(4):711–25.
Wang Y, Dellatore P, Douard V, Qin L, Watford M, Ferraris RP, Lin T, Shapses SA. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr Res. 2016;36(7):742–50.
Zhan Q, Tian Y, Han L, Wang K, Wang J, Xue C. The opposite effects of Antarctic krill oil and arachidonic acid-rich oil on bone resorption in ovariectomized mice. Food Funct. 2020;11(8):7048–60.
Niu Y, Yang Z, Li X, Zhang W, Lu S, Zhang H, Chen X, Zhu L, Xing Y, Ning G, Qin L, Su Q. Association of osteoprotegerin with impaired glucose regulation and microalbuminuria: the REACTION study. BMC Endocr Disord. 2015;15:75.
Guntur AR, Gerencser AA, Le PT, DeMambro VE, Bornstein SA, Mookerjee SA, et al. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. J Bone Miner Res. 2018;33(6):1052–65.
Hahn TJ, Westbrook SL, Sullivan TL, Goodman WG, Halstead LR. Glucose transport in osteoblast-enriched bone explants: characterization and insulin regulation. J Bone Miner Res. 1988;3(3):359–65.
Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, Takarada T, Iezaki T, Pessin JE, Hinoi E, Karsenty G. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–91.
Dirckx N, Tower RJ, Mercken EM, Vangoitsenhoven R, Moreau-Triby C, Breugelmans T, Nefyodova E, Cardoen R, Mathieu C, van der Schueren B, Confavreux CB, Clemens TL, Maes C. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J Clin Invest. 2018;128(3):1087–105.
Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care. 2018;41(7):1526–34.
Pellegrini M, Cioffi I, Evangelista A, Ponzo V, Goitre I, Ciccone G, Ghigo E, Bo S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):17–33.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Puzziferri N, Roshek TB 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42.
Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biologyʼs response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R581–600.
Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, MacLean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1117–29.
MacLean PS, Blundell JE, Mennella JA, Batterham RL. Biological control of appetite: a daunting complexity. Obesity (Silver Spring). 2017;25(Suppl 1):S8–S16.
Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587(Pt 20):4949–61.
MacLean PS, Higgins JA, Giles ED, Sherk VD, Jackman MR. The role for adipose tissue in weight regain after weight loss. Obes Rev. 2015;16(Suppl 1):45–54.
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–71.
Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14(1):10–9.
Ricci C, Cova M, Kang YS, Yang A, Rahmouni A, Scott WW Jr, Zerhouni EA. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology. 1990;177(1):83–8.
Sieron D, Drakopoulos D, Loebelenz LI, Schroeder C, Ebner L, Obmann VC, et al. Correlation between fat signal ratio on T1-weighted MRI in the lower vertebral bodies and age, comparing 1.5-T and 3-T scanners. Acta Radiol Open. 2020;9(1):2058460120901517.
• Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C, et al. Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nat Commun. 2020;11(1):3097. This paper observed differences between bone marrow adipose tissue (BMA) and white and brown adipose tissue in glucose metabolism, thus providing support to the hypothesis of BMA contributing to whole body energy uptake.
• Hussain MM, Mahley RW, Boyles JK, Lindquist PA, Brecht WJ, Innerarity TL. Chylomicron metabolism. Chylomicron uptake by bone marrow in different animal species. J Biol Chem. 1989;264(30):17931-8. This paper is one of the first studies that demonstrates chylomicron uptake into the bone marrow, providing evidence that bone is a metabolically active organ.
Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, Xie Z, Zong X, Styner MA, Rubin CT, Rubin J. Exercise decreases marrow adipose tissue through ss-oxidation in obese running mice. J Bone Miner Res. 2017;32(8):1692–702.
•• McGrath C, Sankaran JS, Misaghian-Xanthos N, Sen B, Xie Z, Styner MA, et al. Exercise degrades bone in caloric restriction, despite suppression of marrow adipose tissue (MAT). J Bone Miner Res. 2020;35(1):106-15. This paper observed increases in bone fatty acid uptake in calorie-restricted mice when compared to mice on a regular diet.
Spurny M, Jiang Y, Sowah SA, Schubel R, Nonnenmacher T, Bertheau R, et al. Changes in bone marrow fat upon dietary-induced weight loss. Nutrients. 2020;12(5).
Scheller EL, Khoury B, Moller KL, Wee NK, Khandaker S, Kozloff KM, et al. Changes in skeletal integrity and marrow adiposity during high-fat diet and after weight loss. Front Endocrinol (Lausanne). 2016;7:102.
Bosy-Westphal A, Later W, Schautz B, Lagerpusch M, Goele K, Heller M, Glüer CC, Müller MJ. Impact of intra- and extra-osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obesity (Silver Spring). 2011;19(7):1503–10.
Kim TY, Schwartz AV, Li X, Xu K, Black DM, Petrenko DM, Stewart L, Rogers SJ, Posselt AM, Carter JT, Shoback DM, Schafer AL. Bone marrow fat changes after gastric bypass surgery are associated with loss of bone mass. J Bone Miner Res. 2017;32(11):2239–47.
Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90.
Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36.
Levental KR, Surma MA, Skinkle AD, Lorent JH, Zhou Y, Klose C, et al. Omega-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Sci Adv. 2017;3(11):eaao1193.
Al Saedi A, C AG, D EM, Hayes A, Duque G. Rapamycin affects palmitate-induced lipotoxicity in osteoblasts by modulating apoptosis and autophagy. J Gerontol A Biol Sci Med Sci. 2020;75(1):58-63.
Jackman MR, Kramer RE, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2006;291(5):E1083–91.
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–19.
Jansson JO, Palsdottir V, Hagg DA, Schele E, Dickson SL, Anesten F, et al. Body weight homeostat that regulates fat mass independently of leptin in rats and mice. Proc Natl Acad Sci U S A. 2018;115(2):427–32.
Ohlsson C, Gidestrand E, Bellman J, Larsson C, Palsdottir V, Hagg D, et al. Increased weight loading reduces body weight and body fat in obese subjects - a proof of concept randomized clinical trial. EClinicalMedicine. 2020;22:100338.
Turner RT, Branscum AJ, Wong CP, Iwaniec UT, Morey-Holton E. Studies in microgravity, simulated microgravity and gravity do not support a gravitostat. J Endocrinol. 2020;247(3):273–82.
Foright RM, Presby DM, Sherk VD, Kahn D, Checkley LA, Giles ED, Bergouignan A, Higgins JA, Jackman MR, Hill JO, MacLean PS. Is regular exercise an effective strategy for weight loss maintenance? Physiol Behav. 2018;188:86–93.
Funding
NIH KL2 TR002534 (VS), ASBMR Rising Star Award (VS)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have any further conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer
This article was prepared while Vanessa Sherk, PhD, was employed at the University of Colorado. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition, Exercise and Lifestyle
Rights and permissions
About this article
Cite this article
Bermudez, B., Ishii, T., Wu, YH. et al. Energy Balance and Bone Health: a Nutrient Availability Perspective. Curr Osteoporos Rep 21, 77–84 (2023). https://doi.org/10.1007/s11914-022-00765-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00765-4