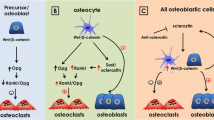
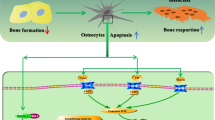
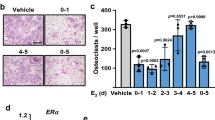
Abstract
Purpose of Review
Postmenopausal osteoporosis reduces circulating estrogen levels, which leads to osteoclast resorption, bone loss, and fracture. This review addresses emerging evidence that osteoporosis is not simply a disease of bone loss but that mechanosensitive osteocytes that regulate both osteoclasts and osteoblasts are also impacted by estrogen deficiency.
Recent Findings
At the onset of estrogen deficiency, the osteocyte mechanical environment is altered, which coincides with temporal changes in bone tissue composition. The osteocyte microenvironment is also altered, apoptosis is more prevalent, and hypermineralization occurs. The mechanobiological responses of osteocytes are impaired under estrogen deficiency, which exacerbates osteocyte paracrine regulation of osteoclasts.
Summary
Recent research reveals changes in osteocytes during estrogen deficiency that may play a critical role in the etiology of the disease. A paradigm change for osteoporosis therapy requires an advanced understanding of such changes to establish the efficacy of osteocyte-targeted therapies to inhibit resorption and secondary mineralization.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104(40):15941–6. https://doi.org/10.1073/pnas.0707246104.
Wang L, Wang Y, Han Y, Henderson SC, Majeska RJ, Weinbaum S, Schaffler MB. In situ measurement of solute transport in the bone lacunar-canalicular system. Proc Natl Acad Sci U S A. 2005;102(33):11911–6. https://doi.org/10.1073/pnas.0505193102.
Knothe Tate ML. “Whither flows the fluid in bone?” An osteocyte’s perspective. J Biomech. 2003;36(10):1409–24. https://doi.org/10.1016/S0021-9290(03)00123-4.
Vaughan TJ, Mullen CA, Verbruggen SW, McNamara LM. Bone cell mechanosensation of fluid flow stimulation: A fluid-structure interaction model characterising the role integrin attachments and primary cilia. Biomech Model Mechanobiol. 2015;14(4):703–18. https://doi.org/10.1007/s10237-014-0631-3.
Verbruggen SW, Vaughan TJ, McNamara LM. Fluid flow in the osteocyte mechanical environment: A fluid-structure interaction approach. Biomech Model Mechanobiol. 2014;13(1):85–97. https://doi.org/10.1007/s10237-013-0487-y.
Verbruggen SW, Vaughan TJ, McNamara LM. Strain amplification in bone mechanobiology: A computational investigation of the in vivo mechanics of osteocytes. J R Soc Interface. 2012;9(75):2735–44. https://doi.org/10.1098/rsif.2012.0286.
Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13–27. https://doi.org/10.22203/ecm.v023a02.
Li J, Rose E, Frances D, Sun Y, You L. Effect of oscillating fluid flow stimulation on osteocyte mRNA expression. J Biomech. 2012;45:247–51.
Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104(33):13325–30. https://doi.org/10.1073/pnas.0700636104.
Lee KL, Hoey DA, Spasic M, Tang T, Hammond HK, Jacobs CR. Adenylyl cyclase 6 mediates loading-induced bone adaptation in vivo. FASEB J. 2014;28(3):1157–65. https://doi.org/10.1096/fj.13-240432.
Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun. 2011;412(1):182–7. https://doi.org/10.1016/j.bbrc.2011.07.072.
Geoghegan IP, McNamara LM, Hoey DA. Estrogen withdrawal alters cytoskeletal and primary ciliary dynamics resulting in increased Hedgehog and osteoclastogenic paracrine signalling in osteocytes. Sci Rep. 2021;11(1):9272. https://doi.org/10.1038/s41598-021-88633-6.
Geoghegan IP, Hoey DA, McNamara LM. Estrogen deficiency impairs integrin alphavbeta3-mediated mechanosensation by osteocytes and alters osteoclastogenic paracrine signalling. Sci Rep. 2019;9(1):4654. https://doi.org/10.1038/s41598-019-41095-3.
Deepak V, Kayastha P, McNamara LM. Estrogen deficiency attenuates fluid flow-induced [Ca2+]i oscillations and mechanoresponsiveness of MLO-Y4 osteocytes. FASEB J. 2017;31:3027–39. https://doi.org/10.1096/fj.201601280R.
Allison H, Holdsworth G, McNamara LM. Scl-Ab reverts pro-osteoclastogenic signalling and resorption in estrogen deficient osteocytes. BMC Mol Cell Biol. 2020;21(1):78. https://doi.org/10.1186/s12860-020-00322-w.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: How osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176–90. https://doi.org/10.1002/dvdy.20603.
Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10(2):118–25. https://doi.org/10.1007/s11914-012-0105-4.
Mullen CA, Haugh MG, Schaffler MB, Majeska RJ, McNamara LM. Osteocyte differentiation is regulated by extracellular matrix stiffness and intercellular separation. J Mech Behav Biomed Mater. 2013;28:183–94. https://doi.org/10.1016/j.jmbbm.2013.06.013.
Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, Gludovatz B, Walsh F, Regan JN, Messina S, Evans DS, Lang TF, Zhang B, Ritchie RO, Mohammad KS, Alliston T. Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21(9):2585–96. https://doi.org/10.1016/j.celrep.2017.10.115.
Bonewald L. Osteocyte remodeling of their perilacunar/canalicular matrix: Hormonal/mechanical regulation. Bone. 2010;46:S11-S. https://doi.org/10.1016/j.bone.2010.01.010.
Milovanovic P, Zimmermann EA, Vom Scheidt A, Hoffmann B, Sarau G, Yorgan T, et al. The formation of calcified nanospherites during micropetrosis represents a unique mineralization mechanism in aged human bone. Small. 2017;13(3):10.1002/smll.201602215.
Frost HM. Micropetrosis. J Bone Joint Surgery-Ame Vol. 1960;42(1):144–50. https://doi.org/10.2106/00004623-196042010-00012.
Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39(9):1735–43. https://doi.org/10.1016/j.jbiomech.2005.04.032.
Verbruggen SW, Mc Garrigle MJ, Haugh MG, Voisin MC, McNamara LM. Altered mechanical environment of bone cells in an animal model of short- and long-term osteoporosis. Biophys J. 2015;108(7):1587–98. https://doi.org/10.1016/j.bpj.2015.02.031.
Knothe Tate ML, Knothe U, Niederer P. Experimental elucidation of mechanical load-induced fluid flow and its potential role in bone metabolism and functional adaptation. Am J Med Sci. 1998;316(3):189–95.
Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22(2):107–17. https://doi.org/10.1016/s8756-3282(97)00234-2.
Price C, Zhou X, Li W, Wang L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: Direct evidence for load-induced fluid flow. J Bone Miner Res. 2011;26(2):277–85. https://doi.org/10.1002/jbmr.211.
Wang Y, McNamara LM, Schaffler MB, Weinbaum S. Strain amplification and integrin based signaling in osteocytes. J Musculoskelet Neuronal Interact. 2008;8(4):332–4.
Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004;101(47):16689–94. https://doi.org/10.1073/pnas.0407429101.
Verbruggen SW, Vaughan TJ, McNamara LM. Mechanisms of osteocyte stimulation in osteoporosis. J Mech Behav Biomed Mater. 2016;62:158–68. https://doi.org/10.1016/j.jmbbm.2016.05.004.
Vaughan TJ, Verbruggen SW, McNamara LM. Are all osteocytes equal? Multiscale modelling of cortical bone to characterise the mechanical stimulation of osteocytes. Int J numer Method Biomed Eng. 2013;29(12):1361–72. https://doi.org/10.1002/cnm.2578.
van Tol AF, Schemenz V, Wagermaier W, Roschger A, Razi H, Vitienes I, Fratzl P, Willie BM, Weinkamer R. The mechanoresponse of bone is closely related to the osteocyte lacunocanalicular network architecture. Proc Natl Acad Sci U S A. 2020;117(51):32251–9. https://doi.org/10.1073/pnas.2011504117.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: An endocrine cell ... and more. Endocr Rev. 2013;34(5):658–90. https://doi.org/10.1210/er.2012-1026.
Guo D, Keightley A, Guthrie J, Veno PA, Harris SE, Bonewald LF. Identification of osteocyte-selective proteins. Proteomics. 2010;10(20):3688–98. https://doi.org/10.1002/pmic.201000306.
Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. https://doi.org/10.1038/s41413-018-0019-6.
Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: Master orchestrators of bone. Calcif Tissue Int. 2014;94(1):5–24. https://doi.org/10.1007/s00223-013-9790-y.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. https://doi.org/10.1002/jbmr.320.
Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol. 2004;36(1):1–8. https://doi.org/10.1016/s1357-2725(03)00241-3.
Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54(2):182–90. https://doi.org/10.1016/j.bone.2012.10.013.
Ishihara Y, Sugawara Y, Kamioka H, Kawanabe N, Hayano S, Balam TA, Naruse K, Yamashiro T. Ex vivo real-time observation of Ca 2+ signaling in living bone in response to shear stress applied on the bone surface. Bone. 2013;53(1):204–15.
Ishihara Y, Sugawara Y, Kamioka H, Kawanabe N, Kurosaka H, Naruse K, Yamashiro T. In situ imaging of the autonomous intracellular Ca 2+ oscillations of osteoblasts and osteocytes in bone. Bone. 2012;50(4):842–52.
Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, DeSimone D, Bonewald LF, Lafer EM, Sprague E, Schwartz MA, Jiang JX. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109(9):3359–64. https://doi.org/10.1073/pnas.1115967109.
Litzenberger JB, Kim JB, Tummala P, Jacobs CR. Beta1 integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif Tissue Int. 2010;86(4):325–32. https://doi.org/10.1007/s00223-010-9343-6.
Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, Yoshimoto Y, Ishikawa H, Chihara K, Takano-Yamamoto T, Fujita T, Mikuni-Takagaki Y. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab. 2006;24(6):498–504. https://doi.org/10.1007/s00774-006-0716-x.
Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9(5):441–5. https://doi.org/10.1096/fasebj.9.5.7896017.
Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes--a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225(1):62–8. https://doi.org/10.1006/bbrc.1996.1131.
Santos A, Bakker AD, Zandieh-Doulabi B, Semeins CM, Klein-Nulend J. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res. 2009;27:1280–7. https://doi.org/10.1002/jor.20888.
Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. https://doi.org/10.1016/j.bone.2016.10.007.
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900. https://doi.org/10.1371/journal.pone.0025900.
Poole KES, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19(10):1842-+. https://doi.org/10.1096/fj.05-4221fje.
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30(12):3071–85. https://doi.org/10.1128/Mcb.01428-09.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–75. https://doi.org/10.1074/jbc.M705092200.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. https://doi.org/10.1038/nm.2452.
Goldring SR. The osteocyte: Key player in regulating bone turnover. RMD Open. 2015;1(Suppl 1):1–4. https://doi.org/10.1136/rmdopen-2015-000049.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41. https://doi.org/10.1038/nm.2448.
Tu XL, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–17. https://doi.org/10.1016/j.bone.2011.10.025.
You L, Temiyasathit S, Lee P, Hyun C, Tummala P, Yao W, et al. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–9. https://doi.org/10.1016/j.bone.2007.09.047.
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif Tissue Int. 2010;87(5):461–8. https://doi.org/10.1007/s00223-010-9407-7.
Kogawa M, Khalid KA, Wijenayaka AR, Ormsby RT, Evdokiou A, Anderson PH, Findlay DM, Atkins GJ. Recombinant sclerostin antagonizes effects of ex vivo mechanical loading in trabecular bone and increases osteocyte lacunar size. Am J Phys Cell Phys. 2018;314(1):C53–61. https://doi.org/10.1152/ajpcell.00175.2017.
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23(4):1225–34. https://doi.org/10.1007/s00198-011-1656-4.
Papanicolaou SE, Phipps RJ, Fyhrie DP, Genetos DC. Modulation of sclerostin expression by mechanical loading and bone morphogenetic proteins in osteogenic cells. Biorheology. 2009;46(5):389–99. https://doi.org/10.3233/BIR-2009-0550.
Thompson WR, Uzer G, Brobst KE, Xie Z, Sen B, Yen SS, Styner M, Rubin J. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci Rep. 2015;5:11049. https://doi.org/10.1038/srep11049.
Hemmatian H, Jalali R, Semeins CM, Hogervorst JMA, van Lenthe GH, Klein-Nulend J, Bakker AD. Mechanical loading differentially affects osteocytes in fibulae from lactating mice compared to osteocytes in virgin mice: Possible role for lacuna size. Calcif Tissue Int. 2018;103(6):675–85. https://doi.org/10.1007/s00223-018-0463-8.
Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, Barry KJ, Uda Y, Lai F, Dedic C, Balcells-Camps M, Kronenberg HM, Babij P, Pajevic PD. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem. 2015;290(27):16744–58. https://doi.org/10.1074/jbc.M114.628313.
Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107(31):13648–53. https://doi.org/10.1073/pnas.1009382107.
Wang B, Lai X, Price C, Thompson WR, Li W, Quabili TR, Tseng WJ, Liu XS, Zhang H, Pan J, Kirn-Safran CB, Farach-Carson MC, Wang L. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2014;29(4):878–91. https://doi.org/10.1002/jbmr.2105.
Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278(44):43146–56. https://doi.org/10.1074/jbc.M302993200.
Maycas M, Ardura JA, de Castro LF, Bravo B, Gortazar AR, Esbrit P. Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. J Bone Miner Res. 2015;30(7):1231–44. https://doi.org/10.1002/jbmr.2439.
Lyons JS, Joca HC, Law RA, Williams KM, Kerr JP, Shi G, et al. Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Sci Signal. 2017;10(506). https://doi.org/10.1126/scisignal.aan5748.
Yu K, Sellman DP, Bahraini A, Hagan ML, Elsherbini A, Vanpelt KT, Marshall PL, Hamrick MW, McNeil A, McNeil PL, McGee-Lawrence ME. Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J Orthop Res. 2018;36(2):653–62. https://doi.org/10.1002/jor.23665.
Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24(8):2859–68. https://doi.org/10.1096/fj.09-148007.
Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281(41):30884–95. https://doi.org/10.1074/jbc.M604772200.
Loiselle AE, Jiang JX, Donahue HJ. Gap junction and hemichannel functions in osteocytes. Bone. 2013;54(2):205–12. https://doi.org/10.1016/j.bone.2012.08.132.
Haugh MG, Vaughan TJ, McNamara LM. The role of integrin alpha(V)beta(3) in osteocyte mechanotransduction. J Mech Behav Biomed Mater. 2015;42:67–75. https://doi.org/10.1016/j.jmbbm.2014.11.001.
Hagan ML, Balayan V, McGee-Lawrence ME. Plasma membrane disruption (PMD) formation and repair in mechanosensitive tissues. Bone. 2021;115970:115970. https://doi.org/10.1016/j.bone.2021.115970.
Gould NR, Torre OM, Leser JM, Stains JP. The cytoskeleton and connected elements in bone cell mechano-transduction. Bone. 2021;115971:115971. https://doi.org/10.1016/j.bone.2021.115971.
McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec (Hoboken). 2009;292(3):355–63. https://doi.org/10.1002/ar.20869.
Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2(10):1132–6.
Michael H, Harkonen PL, Vaananen HK, Hentunen TA. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res. 2005;20(12):2224–32. https://doi.org/10.1359/JBMR.050803.
Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27(3):535–45. https://doi.org/10.1038/sj.emboj.7601984.
Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB. Pro-survival effects of 17beta-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem. 2012;287(2):978–88. https://doi.org/10.1074/jbc.M111.294959.
Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem. 2005;280(8):7317–25. https://doi.org/10.1074/jbc.M412817200.
Joshua J, Kalyanaraman H, Marathe N, Pilz RB. Nitric oxide as a mediator of estrogen effects in osteocytes. Vitam Horm. 2014;96:247–63. https://doi.org/10.1016/B978-0-12-800254-4.00010-6.
Jagger CJ, Chow JW, Chambers TJ. Estrogen suppresses activation but enhances formation phase of osteogenic response to mechanical stimulation in rat bone. J Clin Invest. 1996;98(10):2351–7. https://doi.org/10.1172/JCI119047.
Cheng MZ, Zaman G, Rawlinson SC, Suswillo RF, Lanyon LE. Mechanical loading and sex hormone interactions in organ cultures of rat ulna. J Bone Miner Res. 1996;11(4):502–11. https://doi.org/10.1002/jbmr.5650110411.
Bakker AD, Klein-Nulend J, Tanck E, Albers GH, Lips P, Burger EH. Additive effects of estrogen and mechanical stress on nitric oxide and prostaglandin E2 production by bone cells from osteoporotic donors. Osteoporos Int. 2005;16(8):983–9. https://doi.org/10.1007/s00198-004-1785-0.
Allison H, McNamara LM. Inhibition of osteoclastogenesis by mechanically stimulated osteoblasts is attenuated during estrogen deficiency. Am J Phys Cell Phys. 2019;317(5):C969–C82. https://doi.org/10.1152/ajpcell.00168.2019.
Jia J, Zhou H, Zeng X, Feng S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol Med Rep. 2017;15(4):1539–46. https://doi.org/10.3892/mmr.2017.6168.
Yeh CR, Chiu JJ, Lee CI, Lee PL, Shih YT, Sun JS, Chien S, Cheng CK. Estrogen augments shear stress-induced signaling and gene expression in osteoblast-like cells via estrogen receptor-mediated expression of beta1-integrin. J Bone Miner Res. 2010;25(3):627–39. https://doi.org/10.1359/jbmr.091008.
Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, et al. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res. 2006;21(8):1297–306. https://doi.org/10.1359/jbmr.060504.
Ren J, Wang XH, Wang GC, Wu JH. 17 beta Estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells. Bone. 2013;53(2):587–96. https://doi.org/10.1016/j.bone.2012.12.004.
Rosen CJ. Pathogenesis of osteoporosis. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(2):181–93.
McNamara LM, Ederveen AG, Lyons CG, Price C, Schaffler MB, Weinans H, et al. Strength of cancellous bone trabecular tissue from normal, ovariectomized and drug-treated rats over the course of ageing. Bone. 2006;39(2):392–400. https://doi.org/10.1016/j.bone.2006.02.070.
Brennan MA, Gleeson JP, Browne M, O'Brien FJ, Thurner PJ, McNamara LM. Site specific increase in heterogeneity of trabecular bone tissue mineral during oestrogen deficiency. Eur Cell Mater. 2011;21:396–406. https://doi.org/10.22203/ecm.v021a30.
Brennan MA, Gleeson JP, O'Brien FJ, McNamara LM. Effects of ageing, prolonged estrogen deficiency and zoledronate on bone tissue mineral distribution. J Mech Behav Biomed Mater. 2014;29:161–70. https://doi.org/10.1016/j.jmbbm.2013.08.029.
Busse B, Hahn M, Soltau M, Zustin J, Püschel K, Duda GN, Amling M. Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: Mineralization, morphology and biomechanics of human single trabeculae. Bone. 2009;45(6):1034–43. https://doi.org/10.1016/j.bone.2009.08.002.
Parle E, Tio S, Behre A, Carey JJ, Murphy CG, O'Brien TF, et al. Bone mineral is more heterogeneously distributed in the femoral heads of osteoporotic and diabetic patients: A pilot study. JBMR Plus. 2019:e10253-e. https://doi.org/10.1002/jbm4.10253.
Bousson V, Bergot C, Wu Y, Jolivet E, Zhou LQ, Laredo JD. Greater tissue mineralization heterogeneity in femoral neck cortex from hip-fractured females than controls. A microradiographic study. Bone. 2011;48(6):1252–9. https://doi.org/10.1016/j.bone.2011.03.673.
O'Sullivan LM, Allison H, Parle EE, Schiavi J, McNamara LM. Secondary alterations in bone mineralisation and trabecular thickening occur after long-term estrogen deficiency in ovariectomised rat tibiae, which do not coincide with initial rapid bone loss. Osteoporos Int. 2020;31(3):587–99. https://doi.org/10.1007/s00198-019-05239-5.
Brennan O, Kuliwaba JS, Lee TC, Parkinson IH, Fazzalari NL, McNamara LM, et al. Temporal changes in bone composition, architecture, and strength following estrogen deficiency in osteoporosis. Calcif Tissue Int. 2012;91(6):440–9. https://doi.org/10.1007/s00223-012-9657-7.
Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82(9):3128–35.
Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, Jepsen KJ, Schaffler MB. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46(3):577–83. https://doi.org/10.1016/j.bone.2009.11.006.
Brennan MA, Haugh MG, O'Brien FJ, McNamara LM. Estrogen withdrawal from osteoblasts and osteocytes causes increased mineralization and apoptosis. Horm Metab Res. 2014;46(8):537–45. https://doi.org/10.1055/s-0033-1363265.
Florencio-Silva R, Sasso GRS, Sasso-Cerri E, Simoes MJ, Cerri PS. Effects of estrogen status in osteocyte autophagy and its relation to osteocyte viability in alveolar process of ovariectomized rats. Biomed Pharmacother. 2018;98:406–15. https://doi.org/10.1016/j.biopha.2017.12.089.
Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–75. https://doi.org/10.1016/j.cmet.2007.05.001.
Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Puschel K, Djuric M, et al. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano. 2013;7(9):7542–51. https://doi.org/10.1021/nn401360u.
Mullender MG, Vandermeer DD, Huiskes R, Lips P. Osteocyte density changes in aging and osteoporosis. Bone. 1996;18(2):109–13.
Sharma D, Ciani C, Marin PA, Levy JD, Doty SB, Fritton SP. Alterations in the osteocyte lacunar-canalicular microenvironment due to estrogen deficiency. Bone. 2012;51(3):488–97. https://doi.org/10.1016/j.bone.2012.05.014.
Ciani C, Sharma D, Doty SB, Fritton SP. Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone. Bone. 2014;59:229–34. https://doi.org/10.1016/j.bone.2013.11.026.
Sharma D, Larriera AI, Palacio-Mancheno PE, Gatti V, Fritton JC, Bromage TG, Cardoso L, Doty SB, Fritton SP. The effects of estrogen deficiency on cortical bone microporosity and mineralization. Bone. 2018;110:1–10. https://doi.org/10.1016/j.bone.2018.01.019.
Gatti V, Azoulay EM, Fritton SP. Microstructural changes associated with osteoporosis negatively affect loading-induced fluid flow around osteocytes in cortical bone. J Biomech. 2018;66:127–36. https://doi.org/10.1016/j.jbiomech.2017.11.011.
Voisin M, McNamara LM. Differential beta 1 and beta 3 integrin expression in bone marrow and cortical bone of estrogen deficient rats. Anat Rec (Hoboken). 2015;298:1548–59. https://doi.org/10.1002/ar.23173.
Simfia I, Schiavi J, McNamara LM. Alterations in osteocyte mediated osteoclastogenesis during estrogen deficiency and under ROCK-II inhibition: An in vitro study using a novel postmenopausal multicellular niche model. Exp Cell Res. 2020;392(1):112005. https://doi.org/10.1016/j.yexcr.2020.112005.
Naqvi SM, Panadero Perez JA, Kumar V, Verbruggen ASK, McNamara LM. A novel 3D osteoblast and osteocyte model revealing changes in mineralization and pro-osteoclastogenic paracrine signaling during estrogen deficiency. Front Bioeng Biotechnol. 2020;8:601. https://doi.org/10.3389/fbioe.2020.00601.
Randell KM, Honkanen RJ, Kroger H, Saarikoski S. Does hormone-replacement therapy prevent fractures in early postmenopausal women? J Bone Miner Res. 2002;17(3):528–33.
Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH, Myers SL. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30(2):216–24. https://doi.org/10.1002/jbmr.2351.
Li X, Niu QT, Warmington KS, Asuncion FJ, Dwyer D, Grisanti M, Han CY, Stolina M, Eschenberg MJ, Kostenuik PJ, Simonet WS, Ominsky MS, Ke HZ. Progressive increases in bone mass and bone strength in an ovariectomized rat model of osteoporosis after 26 weeks of treatment with a sclerostin antibody. Endocrinology. 2014;155(12):4785–97. https://doi.org/10.1210/en.2013-1905.
Zhang DY, Hu MY, Chu T, Lin LJ, Wang JY, Li XD, Ke HZ, Qin YX. Sclerostin antibody prevented progressive bone loss in combined ovariectomized and concurrent functional disuse. Bone. 2016;87:161–8. https://doi.org/10.1016/j.bone.2016.02.005.
McClung MR. Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential. Ther Adv Musculoskelet Dis. 2017;9(10):263–70. https://doi.org/10.1177/1759720X17726744.
Holdsworth G, Roberts SJ, Ke HZ. Novel actions of sclerostin on bone. J Mol Endocrinol. 2019;62(2):R167–R85. https://doi.org/10.1530/JME-18-0176.
Holdsworth G, Greenslade K, Jose J, Stencel Z, Kirby H, Moore A, Ke HZ, Robinson MK. Dampening of the bone formation response following repeat dosing with sclerostin antibody in mice is associated with up-regulation of Wnt antagonists. Bone. 2018;107:93–103. https://doi.org/10.1016/j.bone.2017.11.003.
Ominsky MS, Boyce RW, Li X, Ke HZ. Effects of sclerostin antibodies in animal models of osteoporosis. Bone. 2017;96:63–75. https://doi.org/10.1016/j.bone.2016.10.019.
Chavassieux P, Chapurlat R, Portero-Muzy N, Garcia P, Brown JP, Horlait S, et al. Effects of romosozumab in postmenopausal women with osteoporosis after 2 and 12 months: Bone histomorphometry substudy. J Bone Miner Res. 2017;32:S25-S.
Ren Y, Han X, Ho SP, Harris SE, Cao Z, Economides AN, Qin C, Ke H, Liu M, Feng JQ. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB J. 2015;29(7):2702–11. https://doi.org/10.1096/fj.14-265496.
Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu Y, Li J, Qin Y, Sun J, Zheng S, Brown T, Feng JQ, Ke HZ, Bauman WA, Cardozo CC. Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res. 2015;30(11):1994–2004. https://doi.org/10.1002/jbmr.2549.
Achiou Z, Toumi H, Touvier J, Boudenot A, Uzbekov R, Ominsky MS, Pallu S, Lespessailles E. Sclerostin antibody and interval treadmill training effects in a rodent model of glucocorticoid-induced osteopenia. Bone. 2015;81:691–701. https://doi.org/10.1016/j.bone.2015.09.010.
Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, Taketo MM, Burr DB, Plotkin LI, Bellido T. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci. 2015;112(5):E478–E86. https://doi.org/10.1073/pnas.1409857112.
Nioi P, Taylor S, Hu R, Pacheco E, He YDD, Hamadeh H, et al. Transcriptional profiling of laser capture microdissected subpopulations of the osteoblast lineage provides insight into the early response to sclerostin antibody in rats. J Bone Miner Res. 2015;30(8):1457–67. https://doi.org/10.1002/jbmr.2482.
Ominsky MS, Brown DL, Van G, Cordover D, Pacheco E, Frazier E, et al. Differential temporal effects of sclerostin antibody and parathyroid hormone on cancellous and cortical bone and quantitative differences in effects on the osteoblast lineage in young intact rats. Bone. 2015;81:380–91. https://doi.org/10.1016/j.bone.2015.08.007.
Boyce RW, Niu QT, Ominsky MS. Kinetic reconstruction reveals time-dependent effects of romosozumab on bone formation and osteoblast function in vertebral cancellous and cortical bone in cynomolgus monkeys. Bone. 2017;101:77–87. https://doi.org/10.1016/j.bone.2017.04.005.
Taylor S, Ominsky MS, Hu R, Pacheco E, He YD, Brown DL, Aguirre JI, Wronski TJ, Buntich S, Afshari CA, Pyrah I, Nioi P, Boyce RW. Time-dependent cellular and transcriptional changes in the osteoblast lineage associated with sclerostin antibody treatment in ovariectomized rats. Bone. 2016;84:148–59. https://doi.org/10.1016/j.bone.2015.12.013.
Acknowledgements
This publication has emanated from research conducted with the financial support of funding from European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant agreements: No 258992 and No 863795), Science Foundation Ireland (SFI) Grant co-funded under the European Regional Development fund (14/IA/2884), and the Irish Research Council (IRC) under the Laureate Consolidator Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Laoise M. McNamara declares no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteocytes
Rights and permissions
About this article
Cite this article
McNamara, L.M. Osteocytes and Estrogen Deficiency. Curr Osteoporos Rep 19, 592–603 (2021). https://doi.org/10.1007/s11914-021-00702-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00702-x