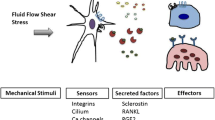
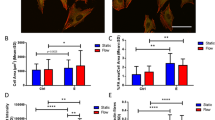
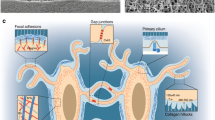
Abstract
Purpose of Review
Osteocytes are the main mechanosensitive cells in bone. Integrin-based adhesions have been shown to facilitate mechanotransduction, and therefore play an important role in load-induced bone formation. This review outlines the role of integrins in osteocyte function (cell adhesion, signalling, and mechanotransduction) and possible role in disease.
Recent Findings
Both β1 and β3 integrins subunits have been shown to be required for osteocyte mechanotransduction. Antagonism of these integrin subunits in osteocytes resulted in impaired responses to fluid shear stress. Various disease states (osteoporosis, osteoarthritis, bone metastases) have been shown to result in altered integrin expression and function.
Summary
Osteocyte integrins are required for normal cell function, with dysregulation of integrins seen in disease. Understanding the mechanism of faulty integrins in disease may aid in the creation of novel therapeutic approaches.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone—the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res 2018;6(1):16. Available from: https://doi.org/10.1038/s41413-018-0019-6 . Review on paracrine and endocrine signalling in bone.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2011;17:1231. Available from. 2011. https://doi.org/10.1038/nm.2452.
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–7 Available from: http://www.jbc.org/content/280/20/19883.abstract.
Holdsworth G, Roberts SJ, Ke HZ. Novel actions of sclerostin on bone. J Mol Endocrinol. 2019;62(2):R167–85 Bristol, UK: Bioscientifica Ltd. Available from: https://jme.bioscientifica.com/view/journals/jme/62/2/JME-18-0176.xml.
Schaffler MB, Cheung W-Y, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94(1):5–24. Available from. https://doi.org/10.1007/s00223-013-9790-y.
Mulvihill BM, McNamara LM, Prendergast PJ. Loss of trabeculae by mechano-biological means may explain rapid bone loss in osteoporosis. J R Soc Interface. 2008;5(27):1243–53 Available from: http://rsif.royalsocietypublishing.org/content/5/27/1243.abstract.
Ihde LL, Forrester DM, Gottsegen CJ, Masih S, Patel DB, Vachon LA, et al. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. RadioGraphics. 2011;31(7):1865–82. Available from:. https://doi.org/10.1148/rg.317115093.
Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, et al. The effect of prolonged physical training on the properties of long bone : a study of Wolff’ s Law. J Bone Jt Surg. 1981;63(5):780–7.
Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–60. Elsevier. Available from:. https://doi.org/10.1016/0021-9290(94)90010-8.
•• Verbruggen SW, Vaughan TJ, McNamara LM. Fluid flow in the osteocyte mechanical environment: a fluid–structure interaction approach Biomech Model Mechanobiol . 2014;13(1):85–97. Available from: https://doi.org/10.1007/s10237-013-0487-y. Describes a computational model of osteocyte mechanical stimulation and highlights the importance of osteocyte cell process in this response.
Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004;101(47):16689–94.
Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20(7):811–27.
Gauthier NC, Roca-Cusachs P. Mechanosensing at integrin-mediated cell–matrix adhesions: from molecular to integrated mechanisms. Curr Opin Cell Biol. 2018;50:20–6 Available from: http://www.sciencedirect.com/science/article/pii/S0955067417301266.
Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci. 2009;122(2):179 LP–186 Available from: http://jcs.biologists.org/content/122/2/179.abstract.
•• Thi MM, Suadicani SO, Schaffler MB, Weinbaum S, Spray DC. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require alphaVbeta3 integrin. Proc Natl Acad Sci U S A. 2013;110(52):21012–7 Describes that α v β 3 integrins along osteocyte cell processes are necessary for mediating fluid flow induced Ca 2+ responses.
•• Haugh MG, Vaughan TJ, McNamara LM. The role of integrin alpha(V)beta(3) in osteocyte mechanotransduction. J Mech Behav Biomed Mater. 2015;42:67–75 Highlights the importance of integrin α v β 3 in osteocyte mechanotransduction in vitro.
Litzenberger JB, Tang WJ, Castillo AB, Jacobs CR. Deletion of β1 integrins from cortical osteocytes reduces load-induced bone formation. Cell Mol Bioeng. 2009;2(3):416–24. Available from. https://doi.org/10.1007/s12195-009-0068-4.
•• Litzenberger JB, Kim JB, Tummala P, Jacobs CR. Beta1 integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif Tissue Int. 2010;86(4):325–32 Highlights the importance of β 1 integrins in osteocyte mechanotransduction in vitro.
• Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38 Review on osteocytes.
Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol. 2004;36(1):1–8.
Hunter RL, Agnew AM. Intraskeletal variation in human cortical osteocyte lacunar density: implications for bone quality assessment. Bone Reports. 2016;5:252–61 Elsevier. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28580394.
Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10(2):118–25. Available from. https://doi.org/10.1007/s11914-012-0105-4.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176–90. John Wiley & Sons, Ltd; Available from. https://doi.org/10.1002/dvdy.20603.
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26(12):4539–52 Available from: https://www.ncbi.nlm.nih.gov/pubmed/16738320.
Delgado-Calle J, Bellido T. Osteocytes and skeletal pathophysiology. Curr Mol Biol Reports. 2015;1(4):157–67 Available from: https://www.ncbi.nlm.nih.gov/pubmed/26693137.
Anderson CT, Castillo AB, Brugmann SA, Helms JA, Jacobs CR, Stearns T. Primary cilia: cellular sensors for the skeleton. Anat Rec (Hoboken). 2008;291(9):1074–8 Available from: https://www.ncbi.nlm.nih.gov/pubmed/18727074.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocr Rev. 2013;34(5):658–90 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23612223.
Lanske B, Densmore MJ, Erben RG. Vitamin D endocrine system and osteocytes. Bonekey Rep. 2014;3:494 Nat Publ Group. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24605211.
Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54(2):182–90 Available from: http://www.sciencedirect.com/science/article/pii/S875632821201321X.
• Hinton P V, Rackard SM, Kennedy OD. In vivo osteocyte mechanotransduction: recent developments and future directions. Curr Osteoporos Rep 2018;16(6):746–753. Available from: https://doi.org/10.1007/s11914-018-0485-1. Review on in vivo osteocyte mechanotransduction.
Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology Netherlands. 2003;40(6):591–603.
• Jacobs C, Temiyasathit S, Castillo A. Osteocyte Mechanobiology and Pericellular Mechanics. Annual review of biomedical engineering. 2010;12(1):369-400. Review on osteocyte mechanobiology.
Geoghegan IP, Hoey DA, McNamara LM. Estrogen deficiency impairs integrin αvβ3-mediated mechanosensation by osteocytes and alters osteoclastogenic paracrine signalling. Sci Rep. 2019;9:4654.
• Deepak V, Kayastha P, McNamara LM. Estrogen deficiency attenuates fluid flow–induced [Ca2+]i oscillations and mechanoresponsiveness of MLO-Y4 osteocytes. FASEB J. 2017; Available from: http://www.fasebj.org/content/early/2017/03/31/fj.201601280R.abstract. Describes the effect of estrogen withdrawal on osteocyte mechanobiology.
Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4(1):7 Available from: http://www.ciliajournal.com/content/4/1/7.
Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24(8):2859–68 Available from: http://www.fasebj.org/content/24/8/2859.abstract.
Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278(44):43146–56 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12939279.
Lyons JS, Joca HC, Law RA, Williams KM, Kerr JP, Shi G, et al. Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Sci Signal. 2017;10(506) Available from: http://stke.sciencemag.org/content/10/506/eaan5748.abstract.
Yu K, Sellman DP, Bahraini A, Hagan ML, Elsherbini A, Vanpelt KT, et al. Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J Orthop Res. 2017 [cited 2017 Sep 29]; Available from:. https://doi.org/10.1002/jor.23665.
Maycas M, Ardura JA, de Castro LF, Bravo B, Gortázar AR, Esbrit P. Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. J Bone Miner Res. 2014;30(7):1231–44. John Wiley & Sons, Ltd Available from. https://doi.org/10.1002/jbmr.2439.
Mullen CA, Vaughan TJ, Billiar KL, McNamara LM. The effect of substrate stiffness, thickness, and cross-linking density on osteogenic cell behavior. Biophys J. 2015;108(7):1604–12 Available from: http://www.sciencedirect.com/science/article/pii/S0006349515001873.
Mullen CA, Haugh MG, Schaffler MB, Majeska RJ, McNamara LM. Osteocyte differentiation is regulated by extracellular matrix stiffness and intercellular separation. J Mech Behav Biomed Mater. 2013;28:183–94 Available from: http://www.sciencedirect.com/science/article/pii/S1751616113002336.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/Sclerostin. J Biol Chem. 2008;283(9):5866–75 Available from: http://www.jbc.org/content/283/9/5866.abstract.
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):4f. Public Library of Science. Available from). https://doi.org/10.1371/journal.pone.0025900.
Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci. 2015;112(5):E478–86 Available from: http://www.pnas.org/content/112/5/E478.abstract.
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, et al. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30(12):3071 LP–3085 Available from: http://mcb.asm.org/content/30/12/3071.abstract.
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23(4):1225–34. Available from. https://doi.org/10.1007/s00198-011-1656-4.
Papanicolaou SE, Phipps RJ, Fyhrie DP, Genetos DC. Modulation of sclerostin expression by mechanical loading and bone morphogenetic proteins in osteogenic cells. Biorheology. 2009;46(5):389–99 Lawrence J. Ellison Musculoskeletal Research Center, Department of Orthopaedic Surgery, University of California at Davis, Sacramento, CA, USA. Available from: http://europepmc.org/abstract/MED/19940355.
Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, et al. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem. 2015;290(27):16744–58 American Society for Biochemistry and Molecular Biology. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25953900.
Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28(4):865–74 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23109229.
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone Elsevier BV. 2012;50(1):209–17.
Macias BR, Aspenberg P, Agholme F. Paradoxical Sost gene expression response to mechanical unloading in metaphyseal bone. Bone. 2013;53(2):515–9 Available from: http://www.sciencedirect.com/science/article/pii/S8756328213000355.
Bakker AD, Klein-Nulend J, Tanck E, Albers GH, Lips P, Burger EH. Additive effects of estrogen and mechanical stress on nitric oxide and prostaglandin E2 production by bone cells from osteoporotic donors. Osteoporos Int. 2005;16(8):983–9. Available from. https://doi.org/10.1007/s00198-004-1785-0.
Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, et al. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J Biol Chem. 2013;288(13):9035–48 Available from: http://www.jbc.org/content/288/13/9035.abstract.
Yeh C-R, Chiu J-J, Lee C-I, Lee P-L, Shih Y-T, Sun J-S, et al. Estrogen augments shear stress–induced signaling and gene expression in osteoblast-like cells via Estrogen receptor–mediated expression of β(1)-integrin. J Bone Miner Res. 2010;25(3):627–39 Wiley Subscription Services, Inc., A Wiley Company; Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3153398/.
Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem. 2005;280(8):7317–25 Available from: http://www.jbc.org/content/280/8/7317.abstract.
Ren J, Wang X-H, Wang G-C, Wu J-H. 17β estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells. Bone. 2013;53(2):587–96 Available from: http://www.sciencedirect.com/science/article/pii/S8756328212014111.
Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB. Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem. 2012;287(2):978–88 Available from: http://www.jbc.org/content/287/2/978.abstract.
Castillo AB, Triplett JW, Pavalko FM, Turner CH. Estrogen receptor-β regulates mechanical signaling in primary osteoblasts. Am J Physiol - Endocrinol Metab. 2014;306(8):E937–44 Bethesda, MD: American Physiological Society; Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3989741/.
Klein-Nulend J, van Oers RFM, Bakker AD, Bacabac RG. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J Biomech. 2015;48(5):855–65 Available from: http://www.sciencedirect.com/science/article/pii/S0021929014006617.
Bonewald LF. Generation and function of osteocyte dendritic processes. J Musculoskelet Neuronal Interact. 2005;5(4):321–324. University of Missouri at Kansas City, Kansas City, MO 64108–2784, USA. bonewaldl@umkc.edu; Available from: http://europepmc.org/abstract/MED/16340122
Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28(2):145–9 Available from: http://www.sciencedirect.com/science/article/pii/S875632820000421X.
•• McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec. 2009;292(3):355–63 Describes distinct α v β 3 integrins sites along osteocyte cell processes in vivo.
•• Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104(40):15941–6 Describes a mathematical model for the role of integrins in osteocyte mechanotransduction.
You L-D, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec Part A Discov Mol Cell Evol Biol. 2004;278A(2):505–13. Wiley Subscription Services, Inc., A Wiley Company; Available from:. https://doi.org/10.1002/ar.a.20050.
•• You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–86 Available from: http://www.sciencedirect.com/science/article/pii/S0021929001001075. Describes the role of the pericellular matrix around osteocyte cell process in the amplification of whole bone strain.
Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun. 2011;412(1):182–7 Elsevier Inc.; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3160132&tool=pmcentrez&rendertype=abstract.
Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, et al. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci. 2010;107(31):13648 LP-13653 Available from: http://www.pnas.org/content/107/31/13648.abstract.
Wang B, Lai X, Price C, Thompson WR, Li W, Quabili TR, et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2014;29(4):878–91. John Wiley & Sons, Ltd. Available from. https://doi.org/10.1002/jbmr.2105.
• Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993;8(5):527–33. https://doi.org/10.1002/jbmr.5650080503 Available from: John Wiley and Sons and The American Society for Bone and Mineral Research (ASBMR); Describes the expression of integrins in human bone.
Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25 Available from: http://www.sciencedirect.com/science/article/pii/009286749290115S.
Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–26 Available from: http://www.sciencedirect.com/science/article/pii/S009286740601405X.
Arnaout MA, Goodman SL, Xiong J-P. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19(5):495–507 Available from: http://www.sciencedirect.com/science/article/pii/S0955067407001196.
Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev. 2007;26(3):567. Available from–78. https://doi.org/10.1007/s10555-007-9078-7.
Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell–matrix adhesion complexes. Biochem Soc Trans. 2004;32(3):416 LP–420 Available from: http://www.biochemsoctrans.org/content/32/3/416.abstract.
• Sun Z, Lambacher A, Fässler R. Nascent adhesions: from fluctuations to a hierarchical organization. Curr Biol. 2014;24(17):R801–3 Available from: http://www.sciencedirect.com/science/article/pii/S0960982214009300. Mini-review on the assembly of focal adhesions.
Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. Available from:. https://doi.org/10.1038/nrm2957.
• Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279(13):12001–4 Available from: http://www.jbc.org/content/279/13/12001.abstract . Mini-review on the role of integrins in mechanotransduction.
Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat Cell Biol. 2002;4:E97. Nature Publishing Group. Available from. https://doi.org/10.1038/ncb0402-e97.
Cary LA, Han DC, Guan JL. Integrin-mediated signal transduction pathways. Histol Histopathol. 1999;14(3):1001–9 Department of Molecular Medicine, Cornell University, Ithaca, NY 14853, USA. Available from: http://europepmc.org/abstract/MED/10425567.
Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9(4):325–42. Available from. https://doi.org/10.1007/s10911-004-1404-x.
Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2009;339(1):269. Available from–80. https://doi.org/10.1007/s00441-009-0834-6.
Inoué S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995;6(12):1619–40 Available from: https://www.ncbi.nlm.nih.gov/pubmed/8590794.
Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84(3):371–9. Elsevier. Available from:. https://doi.org/10.1016/S0092-8674(00)81281-7.
Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–69. Elsevier; Available from. https://doi.org/10.1016/S0092-8674(00)81280-5.
Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21. Nat Publ Group; Available from. https://doi.org/10.1038/nrm2593.
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. Elsevier. Available from. https://doi.org/10.1016/j.ccr.2005.08.010.
Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. 2012;Cell, 151(7):1513–27. Elsevier. Available from. https://doi.org/10.1016/j.cell.2012.11.034.
Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3(4):413–38 Available from: http://www.sciencedirect.com/science/article/pii/S174270610700061X.
Horton MA, Taylor ML, Arnett TR, Helfrich MH. Arg-Gly-Asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res. 1991;195(2):368–75 Department of Haematology, St. Bartholomew’s Hospital, London, United Kingdom.; Available from: http://europepmc.org/abstract/MED/1712731.
Engleman VW, Nickols GA, Ross FP, Horton MA, Griggs DW, Settle SL, et al. A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J Clin Invest. 1997;99(9):2284–92 American Society for Clinical Investigation; Available from: https://www.ncbi.nlm.nih.gov/pubmed/9151803.
Li CF, Ross FP, Cao X, Teitelbaum SL. Estrogen enhances alpha v beta 3 integrin expression by avian osteoclast precursors via stabilization of beta 3 integrin mRNA. Mol Endocrinol. 1995;9(7):805–13 Department of Pathology, Washington University School of Medicine, St. Louis, Missouri 63110, USA.. Available from: http://europepmc.org/abstract/MED/7476964.
Horton MA, Davies J. Perspectives: adhesion receptors in bone J Bone Miner Res. 1989;4(6):803–808. John Wiley & Sons, Ltd; Available from: https://doi.org/10.1002/jbmr.5650040603.
Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;103(1):267 LP–271 Available from: http://jcs.biologists.org/content/103/1/267.abstract.
Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994;9(4):487–96. John Wiley & Sons, Ltd. Available from: https://doi.org/10.1002/jbmr.5650090408.
Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res . John Wiley & Sons, Ltd; Available from. 1997;12(8):1189–97. https://doi.org/10.1359/jbmr.1997.12.8.1189.
•• Cabahug-Zuckerman P, Stout RF, Majeska RJ, Thi MM, Spray DC, Weinbaum S, et al. Potential role for a specialized β3 integrin-based structure on osteocyte processes in bone mechanosensation. J Orthop Res 2017;36(2):642–652. Available from: https://doi.org/10.1002/jor.23792. Wiley-Blackwell; Describes co-localisation of specialised structures around β 3 integrins in osteocytes in vivo.
Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468(7323):580–4. Nat Publ Group; Available from. https://doi.org/10.1038/nature09621.
• Lee D-Y, Li Y-SJ, Chang S-F, Zhou J, Ho H-M, Chiu J-J, et al. Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by αvβ3 and β1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. J Biol Chem. 2010;285(1):30–42 Available from: http://www.jbc.org/content/285/1/30.abstract . Describes downstream signalling following α v β 3 and β 1 integrin activation by mechanical stimulation in osteoblasts.
Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56. Nat Publ Group; Available from–68. https://doi.org/10.1038/nrm1549.
Zhao X, Guan J-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63(8):610–5.
Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313(1):22–37 Available from: https://www.ncbi.nlm.nih.gov/pubmed/17081517.
Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival: evidence for inside-out singaling leading to anoikis. J Biol Chem. 2007;282(33):24120–30 Available from: http://www.jbc.org/content/282/33/24120.abstract.
Nakayamada S, Okada Y, Saito K, Tamura M, Tanaka Y. β1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor κB ligand on osteoblasts and osteoclast maturation. J Biol Chem. 2003;278(46):45368–74.
Lane D, Matte I, Laplante C, Garde-Granger P, Rancourt C, Piché A. Osteoprotegerin (OPG) activates integrin, focal adhesion kinase (FAK), and Akt signaling in ovarian cancer cells to attenuate TRAIL-induced apoptosis. J Ovarian Res. 2013;6(1):82.
• Castillo AB, Blundo JT, Chen JC, Lee KL, Yereddi NR, Jang E, et al. Focal adhesion kinase plays a role in osteoblast mechanotransduction in vitro but does not affect load-induced bone formation in vivo. PLoS One. 2012;7(9):e43291 Public Library of Science. Describes the role of FAK signalling in osteoblast mechanobiology.
Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322. Macmillan Publishers Limited. Available from–30. https://doi.org/10.1038/sj.onc.1204776.
Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–30 Available from: http://www.sciencedirect.com/science/article/pii/S0092867401002689.
Weyts FAA, Li YS, van Leeuwen J, Weinans H, Chien S. ERK activation and alpha v beta 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem. 2002;87(1):85–92 Available from: http://europepmc.org/abstract/MED/12210725.
Tai Y-L, Chen L-C, Shen T-L. Emerging roles of focal adhesion kinase in cancer. Biomed Res Int. Hindawi Publishing Corporation. 2015;2015:690690.
Li S, Kim M, Hu Y-L, Jalali S, Schlaepfer DD, Hunter T, et al. Fluid shear stress activation of focal adhesion kinase: linking to mitogen-activated protein kinases. J Biol Chem. 1997;272(48):30455–62.
le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II–dependent manner. J Cell Biol. 2003;189(7):1107–15 The Rockefeller University Press; Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2894457/.
Yao M, Goult BT, Klapholz B, Hu X, Toseland CP, Guo Y, et al. The mechanical response of Talin. Nat Commun. 2016;7:11966. The Author(s) Available from. https://doi.org/10.1038/ncomms11966.
• Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. John Wiley & Sons, Ltd; 1997;12(12):2014–2023. Available from: https://doi.org/10.1359/jbmr.1997.12.12.2014. Describes the isolation of the MLO-Y4 osteocyte cell line.
Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Physiol. 2005;289(3):C633–43. American Physiological Society; Available from. https://doi.org/10.1152/ajpcell.00278.2004.
Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, et al. Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci. 2012;109(9):3359 LP–3364 Available from: http://www.pnas.org/content/109/9/3359.abstract.
•• Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, et al. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Min Metab. 2006;24(6):498–504 Describes the importance of the integrin α v β 3 in Ca 2+ influx in osteocytes in response to mechanical stimulation.
Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JMA, Klein-Nulend J. Early activation of the β-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun. 2010;391(1):364–9 Available from: http://www.sciencedirect.com/science/article/pii/S0006291X09022396.
Mercurio AM. Lessons from the alpha2 integrin knockout mouse. Am J Pathol. 2002;161(1):3–6 American Society for Investigative Pathology; Available from: https://www.ncbi.nlm.nih.gov/pubmed/12107082.
McNamara LM. Perspective on post-menopausal osteoporosis: establishing an interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. J R Soc Interface. 2010;7(44):353–72 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2842799&tool=pmcentrez&rendertype=abstract.
Miller PD. Denosumab: Anti-RANKL antibody. Curr Osteoporos Rep. 2009;7(1):18–22. Available from. https://doi.org/10.1007/s11914-009-0004-5.
McClung MR. Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential. Ther Adv Musculoskelet Dis. 2017;9(10):263–70 SAGE Publications Available from: https://www.ncbi.nlm.nih.gov/pubmed/28974988.
Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, et al. Integrins α2β1 and α11β1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death. 2011;2:e186. The Author(s) Available from: https://doi.org/10.1038/cddis.2011.71.
•• Voisin M, McNamara LM. Differential β 3 and β 1 integrin expression in bone marrow and cortical bone of estrogen deficient rats. Anat Rec 2015;298(9):1548–1559. Available from: https://doi.org/10.1002/ar.23173 . Describes the expression of β 1 and β 3 integrins in a rat OVX model.
Brennan MA, Haugh MG, O’Brien F, McNamara LM, O’Brien FJ, McNamara LM. Estrogen withdrawal from osteoblasts and osteocytes causes increased mineralization and apoptosis. Horm Metab Res. 2014;46(08):537–45. Available from. https://doi.org/10.1055/s-0033-1363265.
Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. The Author(s); Available from. https://doi.org/10.1038/boneres.2016.44.
Almonte-Becerril M, Costell M, Kouri JB. Changes in the integrins expression are related with the osteoarthritis severity in an experimental animal model in rats. J Orthop Res. 2014;32(9):1161–6. John Wiley & Sons, Ltd; Available from. https://doi.org/10.1002/jor.22649.
Priam S, Bougault C, Houard X, Gosset M, Salvat C, Berenbaum F, et al. Identification of soluble 14-3-3∊ as a novel subchondral bone mediator involved in cartilage degradation in osteoarthritis. Arthritis Rheum. 2013;65(7):1831–42. John Wiley & Sons, ltd Available from. https://doi.org/10.1002/art.37951.
Jaiprakash A, Prasadam I, Feng JQ, Liu Y, Crawford R, Xiao Y. Phenotypic characterization of osteoarthritic osteocytes from the sclerotic zones: a possible pathological role in subchondral bone sclerosis. Int J Biol Sci. 2012;8(3):406–17 Ivyspring International Publisher Available from: https://www.ncbi.nlm.nih.gov/pubmed/22419886.
•• Prasadam I, Farnaghi S, Feng JQ, Gu W, Perry S, Crawford R, et al. Impact of extracellular matrix derived from osteoarthritis subchondral bone osteoblasts on osteocytes: role of integrinβ1 and focal adhesion kinase signaling cues. Arthritis Res Ther. 2013;15(5):R150 BioMed Central Available from: https://www.ncbi.nlm.nih.gov/pubmed/24289792 . Describes the effect of ECM derived from OA patients on MLO-Y4 β 1 integrin expression and FAK signalling.
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone metastases: an overview. Oncol Rev. 2017;11(1):321 PAGEPress Publications, Pavia, Italy; Available from: https://www.ncbi.nlm.nih.gov/pubmed/28584570.
Waning DL, Guise TA. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin Cancer Res. 2014;20(12):3071–7 Available from: https://www.ncbi.nlm.nih.gov/pubmed/24677373.
Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895. Nat Publ Group Available from–904. https://doi.org/10.1038/nm1469.
Jung Y, Wang J, Schneider A, Sun Y-X, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38(4):497–508 Available from: http://www.sciencedirect.com/science/article/pii/S875632820500414X.
Coughlin TR, Romero-Moreno R, Mason DE, Nystrom L, Boerckel JD, Littlepage* GN and LE. Bone: a fertile soil for cancer metastasis . Vol. 18, Current Drug Targets. 2017. p. 1281–95. Available from: http://www.eurekaselect.com/node/148704/article
• Choudhary S, Ramasundaram P, Dziopa E, Mannion C, Kissin Y, Tricoli L, Albanese C, Lee W, Zilberberg J Human ex vivo 3D bone model recapitulates osteocyte response to metastatic prostate cancer. Sci Rep 2018;8(1):17975. Available from: https://doi.org/10.1038/s41598-018-36424-x. Describes the effect of prostate cancer cell-osteocyte co-culture on osteocyte functions.
Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816. Nat Publ Group; Available from–26. https://doi.org/10.1038/nrm1490.
Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin β4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6(5):471–83 Available from: http://www.sciencedirect.com/science/article/pii/S1535610804002995.
Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone. 2011;48(1):54–65 Available from: https://www.ncbi.nlm.nih.gov/pubmed/20850578.
Cui Y, Evans B, Jiang W. New roles of osteocytes in proliferation, migration and invasion of breast and prostate cancer cells. Anticancer Res. 2016;36:1193–201.
• Wang W, Sarazin BA, Kornilowicz G, Lynch ME. Mechanically-loaded breast cancer cells modify osteocyte mechanosensitivity by secreting factors that increase osteocyte dendrite formation and downstream resorption. Front Endocrinol. 2018;9:352 Available from: https://www.frontiersin.org/article/10.3389/fendo.2018.00352 . Describes the effect of conditioned media from mechanically stimulated breast cancer cell line on osteocyte cell processes.
Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(Pt 13):3015–24 The Company of Biologists; Available from: https://www.ncbi.nlm.nih.gov/pubmed/22797912.
Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. Establishment of an osteoid P preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. J Bone Miner Res. 2009;16(9):1622–33. John Wiley & Sons, Ltd; Available from. https://doi.org/10.1359/jbmr.2001.16.9.1622.
Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26(11):2634–46. John Wiley & Sons, Ltd; Available from. https://doi.org/10.1002/jbmr.465.
Divieti P, Inomata N, Chapin K, Singh R, Jüppner H, Bringhurst FR. Receptors for the carboxyl-terminal region of PTH(1–84) are highly expressed in osteocytic cells. Endocrinology. 2001;142(2):916–25. Available from. https://doi.org/10.1210/endo.142.2.7955.
Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–88 Ivyspring International Publisher. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21547158.
Funding
This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) and is co-funded under the European Regional Development Fund under Grant Number 13/RC/2073 and Grant Number 14/IA/2884. The authors received funding from European Research Council (ERC) Starting Grants (336882 and 258992) and Science Foundation Ireland (SFI) Grants 13/ERC/L2864 and 12/RC/2278.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ivor P. Geoghegan, David A. Hoey and Laoise M. McNamara declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteocytes
Rights and permissions
About this article
Cite this article
Geoghegan, I.P., Hoey, D.A. & McNamara, L.M. Integrins in Osteocyte Biology and Mechanotransduction. Curr Osteoporos Rep 17, 195–206 (2019). https://doi.org/10.1007/s11914-019-00520-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-019-00520-2