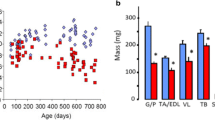
Abstract
Frailty is highly prevalent in the elderly, increasing the risk of poor outcomes that include falls, incident disability, hospitalization, and mortality. Thus, a great need exists to characterize the underlying mechanisms and ultimately identify strategies that prevent, delay, and even reverse frailty. Mouse models can provide insight into molecular mechanisms of frailty by reducing variability in lifestyle and genetic factors that can complicate interpretation of human clinical data. Frailty, generally recognized as a syndrome involving reduced homeostatic reserve in response to physiologic challenges and increasing susceptibility to poor health outcomes, is predominantly assessed using two independent strategies, integrated phenotype and deficit accumulation. The integrated phenotype defines frailty by the presentation of factors affecting functional capacity such as weight loss, exhaustion, low activity levels, slow gait, and grip strength. The deficit accumulation paradigm draws parameters from a greater range of physiological systems, such as the ability to perform daily activities, coordination and gait, mental components, physiological problems, and history and presence of medical morbidities. This strategic division also applies within the emerging field of mouse frailty models, with both methodologies showing usefulness in providing insight into physiologic mechanisms and testing interventions. Our review will explore the strategies used, caveats in methodology, and future directions in the application of animal models for the study of the frailty syndrome.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importancee
Aging, N.I.o., Global health and aging. 2011(NIH Publication no. 11-7737).
Morley JE et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. Report highlighting consensus definition for physical frailty agreed upon by delegates from 6 major international, European and US societies that included both Linda Fried and Kenneth Rockwood.
Choi J et al. Global prevalence of physical frailty by Fried’s criteria in community-dwelling elderly with national population-based surveys. J Am Med Dir Assoc. 2015;16(7):548–50.
Clegg A et al. Frailty in elderly people. Lancet. 2013;381(9868):752–62.
Gielen E et al. Musculoskeletal frailty: a geriatric syndrome at the core of fracture occurrence in older age. Calcif Tissue Int. 2012;91(3):161–77.
Walker SR, Wagner M, Tangri N. Chronic kidney disease, frailty, and unsuccessful aging: a review. J Ren Nutr. 2014;24(6):364–70.
Muhlberg W, Sieber C. Sarcopenia and frailty in geriatric patients: implications for training and prevention. Z Gerontol Geriatr. 2004;37(1):2–8.
Santilli V et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177–80.
Yuan R, Peters LL, Paigen B. Mice as a mammalian model for research on the genetics of aging. ILAR J. 2011;52(1):4–15.
Barreto G, Huang TT, Giffard RG. Age-related defects in sensorimotor activity, spatial learning, and memory in C57BL/6 mice. J Neurosurg Anesthesiol. 2010;22(3):214–9.
Fahlstrom A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging. 2011;32(10):1868–80. Study makes use of novel methodologies in assessing age-related changes in mice that are of use in the study of animal frailty.
Fujita T et al. A high-fat diet delays age-related hearing loss progression in C57BL/6J mice. PLoS One. 2015;10(1):e0117547.
Brooks SV, Faulkner JA. Maximum and sustained power of extensor digitorum longus muscles from young, adult, and old mice. J Gerontol. 1991;46(1):B28–33.
Graber TG et al. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci. 2013;68(11):1326–36. This report makes use of several assessments in the creation of a neuromuscular health index that is useful for the study of animal frailty.
Justice JN et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr). 2014;36(2):583–92. This report makes use of several assessments in the creation of a motor functional health index that is useful for the study of animal frailty.
Ballak SB et al. Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res Rev. 2014;14:43–55.
Fried LP et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. Landmark study establishing the Fried frailty criteria, encompassing unintentional weight loss, exhaustion, weakness, slow walking speed, and low physical activity.
Boeckxstaens P et al. The relationship of multimorbidity with disability and frailty in the oldest patients: a cross-sectional analysis of three measures of multimorbidity in the BELFRAIL cohort. Eur J Gen Pract. 2015;21(1):39–44.
Lin CC et al. Reduced health-related quality of life in elders with frailty: a cross-sectional study of community-dwelling elders in Taiwan. PLoS One. 2011;6(7):e21841.
Rockwood K et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. Study establishing the Rockwood frailty index. This report utilizes an index of 70 parameters of health and aging that include ability to perform daily activities, coordination and gait, mental components, physiological problems, and history and presence of medical morbidities.
Li G et al. Comparison between frailty index of deficit accumulation and phenotypic model to predict risk of falls: data from the global longitudinal study of osteoporosis in women (GLOW) Hamilton cohort. PLoS One. 2015;10(3):e0120144.
Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–7.
Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle-aged African Americans. J Nutr Health Aging. 2012;16(7):601–8.
Garcia-Garcia FJ et al. A new operational definition of frailty: the frailty trait scale. J Am Med Dir Assoc. 2014;15(5):371 e7–371 e13.
Gobbens RJ et al. The Tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–55.
Peters LL et al. Measurement properties of the Groningen frailty indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc. 2012;13(6):546–51.
Parks RJ et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67(3):217–27. First attempt at an animal frailty index in mice from Rockwood and Howlett et al. that utilizes a 31-parameter scale derived from activity monitoring, hemodynamics, body composition, and serum analysis.
Whitehead JC et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69(6):621–32. Second attempt at an animal frailty index from Rockwood and Howlett et al. that utilizes a 31-parameter scale derived from visually inspected elements.
Liu H et al. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2014;69(12):1485–91. Foundational study of an animal frailty phenotype that utilizes rotarod, activity wheels, and a grip endurance inverted-cling device to reach equivalent test used in the Fried frailty criteria.
Borsch-Supan A et al. Data resource profile: the survey of health, ageing and retirement in Europe (SHARE). Int J Epidemiol. 2013;42(4):992–1001.
Theou O et al. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older Europeans. Age Ageing. 2013;42(5):614–9.
Feridooni HA, et al. Reliability of a frailty index based on the clinical assessment of health deficits in male C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2014;70(6):686–93.
Kane AE, et al. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2015. doi:10.1093/gerona/glu315. Validation of a mouse frailty index using resveratrol and caloric restriction as interventions.
Howlett SE, Rockwood K. Factors that influence reliability of the mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2015;70(6):696.
Graber TG, et al. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci. 2014. doi:10.1093/gerona/glu163. Validation of the mouse frailty criteria using voluntary wheel running as an intervention.
Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol. 2012;49(1):85–105.
Hubbard-Turner T, Guderian S, Turner MJ. Lifelong physical activity and knee osteoarthritis development in mice. Int J Rheum Dis. 2015;18(1):33–9.
Yamamoto K et al. Morphological studies on the ageing and osteoarthritis of the articular cartilage in C57 black mice. J Orthop Surg (Hong Kong). 2005;13(1):8–18.
Mohler MJ et al. The Frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol. 2014;54:6–13. Excellent review on current human and animal models of frailty. Reviewers examine potential mechanisms of frailty, compare methodology of assessing frailty between humans and animals, and posit action items for improving understanding, diagnosis, and management of frailty.
Ogbonna AC, Clark AK, Malcangio M. Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice. Age (Dordr). 2015;37(3):9792.
Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci. 2014;281(1786–1791). doi:10.1098/rspb.2014.0210.
Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36(3):1001–14.
Fujibayashi Y et al. Differential aging pattern of cerebral accumulation of radiolabeled glucose and amino acid in the senescence accelerated mouse (SAM), a new model for the study of memory impairment. Biol Pharm Bull. 1994;17(1):102–5.
Walston J et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63(4):391–8.
Compliance with Ethics Guidelines
Conflict of Interest
The authors of this paper declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Muscle and Bone
Rights and permissions
About this article
Cite this article
Seldeen, K.L., Pang, M. & Troen, B.R. Mouse Models of Frailty: an Emerging Field. Curr Osteoporos Rep 13, 280–286 (2015). https://doi.org/10.1007/s11914-015-0283-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-015-0283-y