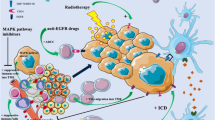
Abstract
Purpose of Review
This review will explore various strategies to rendering MSS mCRCs susceptible to ICI. Moreover, we will provide an overview of potential biomarkers that may aid to better patient selection, and discuss ongoing efforts in this area of research.
Recent Findings
Colorectal cancer (CRC) ranks among the top three most common cancers worldwide. While significant advances in treatment strategies have improved the prognosis for patients in the early stages of the disease, treatment options for metastatic CRC (mCRC) remain limited. Although immune checkpoint inhibitors (ICI) have revolutionized the treatment of several malignancies, its efficacy in mCRC is largely confined to patients exhibiting a high microsatellite instability status (MSI-H). However, the vast majority of mCRC patients do not exhibit a MSI-H, but are microsatellite stable (MSS). In these patients ICIs are largely ineffective.
Summary
So far, ICIs do not play a crucial role in patients with MSS mCRC, despite the promising data for inducing long-term remissions in other tumour entities. For this reason, novel treatment strategies are needed to overcome the primary resistance upon ICI in patients with MSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer globally, and represents the second most common malignant tumor in women and the third most common in men [1]. The prognosis of patients with CRC depends on the stage of disease at initial diagnosis and other clinical and biological risk factors [2]. Regrettably, around 50% of patients are diagnosed at an advanced stage initially [3]. Although great advances in the treatment of advanced or metastasized CRC (mCRC) have been achieved, it remains one of the leading causes of death worldwide [2].
In recent decades, a significant wave of approved targeted therapeutics has been integrated into clinical practice. Understanding molecular signaling networks that control intestinal regeneration and homeostasis has helped to discover key drivers in CRC pathogenesis, progression and novel treatment targets [4]. Molecular profiling and tumor characterization, including chromosomal instability, microsatellite instability (MSI), and the CpG island methylator phenotype, have thus, become essential tools in guiding clinical decision making [3]. While some novel therapies, such as drugs targeting KRAS [5] or BRAF [6] mutations, have shown outstanding clinical improvement, other strategies such as immune checkpoint inhibition (ICI) has only proven to be effective in patients with mCRC that are mismatch-repair-deficient (dMMR) or MSI-High (MSI-H) mCRC [3]. One of the first trials, investigating the efficacy of ICI in dMMR/MSI-H CRCs was published in 2015. Le et al. directed a phase 2 trial aiming to investigate the efficacy of the anti-PD1 checkpoint inhibitor pembrolizumab in patients with mCRC with or without dMMR. They observed that a high somatic mutational load was related to a longer progression-free survival (PFS) (p = 0.02) when treated with ICI, and thus concluded that MMR status might be predict benefit of ICI [7]. The subsequent phase 3 Keynote-177 trial was able to demonstrate a meaningful superiority in terms of response rates, PFS and overall survival (OS) of pembrolizumab in comparison to standard-of-care chemoimmunotherapy, which finally led to the approval of pembrolizumab as a new first-line therapy in mCRC patients with dMMR/MSI-H mCRC [8]. Similar promising results were observed with another anti-PD1 inhibitor (nivolumab), which was primarily investigated in the phase 2 CheckMate-142 trial [9]. The combination of nivolumab and the anti cytotoxic T-lymphocyte-associated protein (CTLA4) inhibitor ipilimumab improved even the results of nivolumab monotherapy [10, 11] in terms of prolonging survival leading to its approval as a second-line treatment option in patients with dMMR/MSI-H mCRC [3]. The subsequent phase 3 CheckMate-8HW trial evaluated the combination of nivolumab and ipilimumab in the first-line setting in mCRCs. The trial met its primary endpoint in showing a prolongation of PFS in the ICI cohort in comparison to standard-of-care chemoimmunotherapy [12]. For this reason, nivolumab plus ipilimumab represent a further first-line treatment option in patients with dMMR/MSI-H mCRC next to pembrolizumab monotherapy.
Although ICI provides a clinically effective and long lasting anti-cancerous response, the majority of mCRC patients are mismatch-repair-proficient (pMMR), microsatellite-stable (MSS), or have low MSI (MSI-L) status, and thus do not benefit from this treatment highlighting the strong need for alternative strategies. The mechanisms of resistance in these tumors, also referred to as “cold” tumors, is an ongoing topic of research. Especially the idea of ICI-based combinational treatments to boost sensitivity to ICI is of great clinical interest [13]. This review will provide a landscape of various current strategies to overcome resistance to ICI and how to change the treatment paradigm in pMMR/MSS mCRC.
Exploring Innovative Treatment Modalities Based on the Latest Research Results
Recent clinical trials have made progress in exploring ICI options for dMMR/MSS mCRC. Table 1 summarises the most recent studies (ongoing, completed and discontinued studies) presented at the most recent oncology conferences in 2023 and 2024. In the following sections several of the listed options will be discussed more in detail.
Immune Checkpoint Inhibitors in Advanced pMMR/MSS mCRC: Monotherapy or Combination Therapy?
The absence of antitumor activity for single-agent ICI in refractory pMMR/MSS mCRC has been substantiated in multiple clinical trials involving nivolumab, pembrolizumab, and the anti-PDL1 inhibitor atezolizumab. For instance, an early pan-tumor basket trial investigating the efficacies of pembrolizumab as well as nivolumab demonstrated no meaningful response rates in pMMR/MSS mCRC patients [7, 14]. Similarly, atezolizumab in comparison with the multi-kinase inhibitor regorafenib failed to show any efficacy in these patients [15]. These unsatisfactory results prompted the investigation of combination therapies. Combination therapies are intended to ensure that chemotherapy strengthens the immune response by increasing the immunogenicity of cancer cells or reducing immunosuppressive mechanisms. Important checkpoints in CRC are lymphocyte-activation gene 3 (LAG3), CTLA4, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) and PDL1, which control tumor growth and progression [16].
The METIMMOX study evaluated the efficacy between FLOX with and without nivolumab in untreated unresectable MSS mCRC. Unfortunately, the addition of nivolumab did not show a significant advantage in terms of PFS. However, in the subgroup of ≥ 60 years patients, the addition of nivolumab showed a significant survival improvement (PFS 13.6 months, p = 0.021), which suggests a potential benefit in this subgroup [17]. The combination of CTLA4 inhibitors with PDL1 inhibitors was investigated in the single arm study MEDTREME [18]. The combination of durvalumab and tremelimumab in combination with mFOLFOX6 was evaluated in RAS-mutated unresectable mCRCs. They set the hypothesis that the FOLFOX regime could trigger immunogenic cell death and eliminate myeloid-derived suppressor cells. The trial showed promising efficacy, with a PFS prolongation of 8.2 months [19]. Additional studies, such as MAIA [20], used the combination of botensilimab (CTLA4 inhibitor) and balstilimab (PDL1 inhibitor), which has shown promising effects in improving T-cell function. With an ORR of 17%, a DCR of 61% and a 12-month OS rate of 60%, the combination therapy has potential efficacy in pMMR/MSS mCRC patients [21]. These combinations show promising results in pMMR/MSS mCRC mCRCs, especially in the subpopulation without liver metastases. Other still ongoing studies which were presented at the major oncology conferences in 2023/24 regarding immunotherapy in pMMR/MSS mCRC are summarised in Table 1.
Immune Checkpoint Inhibitors Combined with Tyrosine-Kinase Inhibitors
Targeted therapies selectively influence molecules involved in cancer cell proliferation, growth and metastasis [22]. Multiple trials have been conducted so far to investigate the optimal combination partners with ICIs. A single-center non-randomized clinical trial [23] (NCT04362839) evaluated the combination of regorafenib, ipilimumab, and nivolumab in MSS mCRC. The RP2D cohort demonstrated an ORR of 36%, DCR of 68% and a PFS of 5.0 months in patients without liver metastases [24]. In the REGONIVO study [25], the combination of regorafenib and nivolumab showed promising data in the 1b trial with then lacking a confirmation of response in the phase 2 CRC only trial [26]. However, the combination of regorafenib with nivolumab increased the response rates and has shown to be able to overcome resistance to ICIs. In the presence of liver metastases, there are some restrictions, such as the lack of synergistic effect and the lack of predictive biomarkers. This treatment could represent a valid therapeutic option for patients with CRC without liver metastases [27].
In BRAFV600E-mutated and MSS mCRCs, a phase I trial [28] investigating the efficacy of cetuximab, camrelizumab, and vemurafenib (VCC) showed an ORR of 40.0% and a DCR of 80.0%. This suggests that VCC holds promise as a treatment option for this specific patient population justifying further investigation [29].
MEK inhibition in preclinical models increased MHC-I expression and the presence of antigen-specific CD8+ T cells in CRC. Combining MEK with PDL1 inhibitors resulted in tumor regression in mouse models in KRAS-mutated CRC [30]. On this basis, the phase III STELLAR-303 [31] study compares zanzalintinib, atezolizumab with regorafenib in mCRC patients with MSS/low-MSI and without liver metastases. Recruitment is still ongoing [32]. Additionally, one of the two randomized trials combining atezolizumab and cobimetinib, a MEK inhibitor, versus regorafenib monotherapy did not show any significant efficacy (IMBlaze370) [15]. Similarly, the LEAP trial, evaluating the multi-kinase inhibitor lenvatinib combined with pembrolizumab failed to demonstrate superior OS compared to standard-of-care chemotherapy in refractory advanced pMMR/MSS mCRC [33].
Immune Checkpoint Inhibitors Combined with Chemotherapy
The combination of chemotherapy and ICI in pMMR/MSS mCRC has yielded conflicting results so far. In later lines of treatment, adding ICI to standard chemotherapy with or without vascular endothelial growth factor (VEGF) inhibitors did not reveal ground-breaking outcomes [34,35,36]. In contrast, studies evaluating VEGF inhibitors and ICIs in the first-line setting showed promising ORR and survival data. The phase II AtezoTRIBE trial compared FOLFOXIRI plus the VEGF antibody bevacizumab with or without atezolizumab and reported a significant improvement in PFS [37]. A single-arm phase I/II study evaluated the addition of the anti-PDL1 inhibitor durvalumab and the anti-CTLA4 inhibitor tremelimumab to first-line FOLFOX regimen in patients with RAS-mutated mCRC observing a PFS of 8.2 months and an ORR of 63% compared to historical controls of FOLFOX without bevacizumab (PFS: 5–6 months; ORR: 36%) [19]. Moreover, the CheckMate 9X8 trial investigated mFOLFOX6 plus bevacizumab combined with nivolumab in the first line, showing higher benefits in the subgroup of TMB-high patients [38].
Despite the lack of trailblazing OS improvements in these combination strategies, new therapeutic approaches are eagerly awaited. A potential near-future practice-changing approach was recently presented at the annual meeting of the American Society of Clinical Oncology (ASCO) in 2024. The ARC-9 phase 2 trial combined etrumadenant, a novel adenosine receptor inhibitor, with zimberelimab, another anti-PD1 antibody, and standard re-challenge FOLFOX plus bevacizumab therapeutics. This combination yielded an ORR of 13%, a PFS of 6.2 months, and an OS of 19.7 months significantly outperforming the control arm with regorafenib. Comparing this mostly third-line population in a cross-trial manner to the new standard third-line treatment of trifluridine/tipiracil plus bevacizumab, the ARC-9 trial reported the most promising survival data in the third-line setting to date [39].
Immunotherapy in Combination with Monoclonal Antibodies
Immunotherapy Plus Anti-EGFR Antibodies
Previous evidence suggests that anti-epidermal growth factor receptor (EGFR) therapies may enhance tumor response and trigger an immunogenic apoptosis cascade that is usually associated with increased expression of CTLA4 and PDL1 [40]. AVETRIC [41], a phase 2 study has investigated the addition of avelumab (PDL1 inhibitor) to the anti-EGFR antibody cetuximab and FOLFOXIRI in the first-line treatment of pMMR/MSS mCRC that do not harbour any mutation in pan-RAS or BRAF genes. It met its primary endpoint and showed promising results with a PFS of 14.1 months and an ORR of 82% [42].
Immunotherapy Plus Antiangiogenic Antibodies
Several studies have shown that antiangiogenic agents, by inhibiting VEGF signaling, normalize tumor vasculature and increase T-cell infiltration, thereby enhancing immune cell activation [43]. Atezolizumab is currently approved for the treatment of metastatic non-small cell lung cancer and advanced urothelial carcinoma [44]. The Atezo-TRIBE [45] study, as already mentioned above, included 218 patients of whom were 92% MSS. In this subgroup, the addition of atezolizumab to bevacizumab and chemotherapy demonstrated an increase in 37 months-PFS (11.5 months (dual) vs 13.0 months (triple)). Furthermore, patients in this trial with a high immunoscore (CD3+ and CD8+ T cells) and/or immunoscore-IC (CD8+ and PDL1+) showed an improved PFS compared to those in their respective low group. Interestingly, the amount of tumor infiltrating lymphocytes correlated poorly with the immunoscore indicating that the presence of special subpopulations of T cells contributes to ICI response [46]. These results show that patients with elevated Immunoscore IC and/or TMB in pMMR/MSS mCRC experience a survival benefit when atezolizumab was added. The combination of chemotherapy and immunotherapy has not yet achieved clinically meaningful results in pMMR/MSS mCRC patients. Further trials are still ongoing.
Immunotherapy in Combination with Radiotherapy
Up to two-thirds of all cancer patients with solid tumors undergo radiation therapy underscoring its pivotal role as a standard-of-care treatment modality. The primary objective of radiotherapy is to achieve local tumor control by inducing DNA damage, resulting in cell death and subsequently enhancing the immune system’s anti-tumor response [47]. Combining radiotherapy with either one or multiple ICIs or with anti-VEGF targeted therapies, may be a promising approach overcoming primary resistance upon ICI.
The FRUIT trial [48] investigated fruquintinib combined with the IgG4 antibody tislelizumab and SBRT in later-line therapy in pMMR/MSS mCRC. It showed a PFS of 5.1 months and among 24 enrolled patients, six achieving partial response and 13 stable disease, resulting in an ORR of 26% and a DCR of 83% [49]. Furthermore, the NEST-1 trial tested the safety and efficacy of neoadjuvant botensilimab and balstilimab in patients with pMMR/dMMR CRCs showing significant tumor regression without delaying surgery or increasing severe adverse events. The positive results suggest that this regimen could potentially downstage tumors reducing the need for surgery and/or adjuvant chemotherapy independent of MMR/MSS status [50]. Currently, a further study has been initiated to further explore dosing and timing of this ICI combination [51].
Immunotherapy in Combination with Vaccine
One treatment method that has garnered considerable interest in recent years is cancer vaccine therapy. These vaccines target individual peptides of antigens released by tumor cells and trigger the process of activating the immune response [52]. Certain cytotoxic drugs have been described to have immunomodulatory effects and may act synergistically with cancer vaccines by enhancing their antitumor activity. Recently, nano- and neoantigen vaccines have emerged as a potential strategy to overcome the resistance upon ICI [53]. However, if this approach will enter clinical practice remains to be elucidated.
Conclusion
While ICI monotherapies show only limited efficacy, innovative combinations with targeted therapies, chemotherapy and radiotherapy are possible combinations in patients with pMMR/MSS mCRC. So far, the most promising results have been achieved by combining different ICIs, which deepen the responses when combined with radiotherapy, especially in localized rectal cancer. Nevertheless, further modulation of the tumor microenvironment (TME) and the identification of predictive biomarkers will be crucial for future developments which will be addressed in the next chapter.
Biomarkers for Patient Selection
While previous sections have been focused on methods how to transform therapeutically „cold “ in „hot “ tumors, in this section we are focusing on biomarkers that have been evaluated to provide additional guidance to find a subset of pMMR/MSS mCRC patients who might benefit from ICIs. Despite these efforts, no clear conclusions can be drawn based on the data currently available. Herein, we are summarizing the most popular clinical and molecular biomarkers predicting ICI response.
Programmed-Death Ligand 1 Expression
Programmed-death ligand 1 (PDL1) expression has been extensively evaluated in CRC using immunohistochemistry (IHC) [54,55,56]. The prevalence of PDL1 IHC expression in CRC varies significantly [56]. Although, PDL1 IHC expression is a routinely used biomarker for predicting ICI response for multiple other malignancies, such as lung or gastric cancer, this has not been the case for CRC, as demonstrated for instance in sub-analysis of the CheckMate-9 × 8 trial [38]. For this reason, PDL1 IHC status is currently not sufficiently validated to predict ICI response in mCRC patients. Therefore, additional predictive biomarkers are needed to select the right patient who might derive the greatest benefit from ICI.
Tumor Mutational Burden
Tumor mutational burden (TMB) may serve as a viable metric to assess damage on a genomic level and may have implications for choice of treatment regimen. About 3% of all patients with CRC who harbour a MSS status, have a high-TMB. Definitions of high-TMB vary widely, from > 9 to > 37 mut/Mb, complicating comparisons across trials and tumor entities [57,58,59]. To facilitate comparisons between studies, experts and stakeholders defined a high-TMB tumor when TMB levels are > 10 mut/Mb [60]. Pembrolizumab, also FDA-approved for the treatment of high-TMB tumors, may be a good option in high-TMB mCRCs, irrespective of MSS status [61].
DNA Polymerase Epsilon/Delta1 Mutations
DNA polymerase epsilon/delta1 (POLE/POLD1) mutations have shown to be associated with an improved outcome upon ICI in various tumor entities [62]. These mutations occur in about 1% of pMMR/MSS CRC patients [63] causing, regardless of the MSS status, hypermutations due to defects in the nucleotide and base excision repair system [64]. Wang et al. investigated different types of solid tumors treated with ICI with a special emphasis on POLE and POLD1 mutations, wherein 75% of patients were pMMR/MSS. While POLE/POLD1 mutations were detectable in around 2–3% in all malignancies, the prevalence was higher in CRC with a mutational rate found close to 7%. Moreover, their results revealed a significant survival benefit for ICI-treated patients; more than doubling the OS when comparing them to their non-mutated counterparts [65]. Only very recently, in a retrospective study, investigators analyzed data from 27 mCRC patients harboring POLE/POLD1 mutations who were treated with ICI and compared them with a historical dMMR/MSI-H cohort [66]. The study revealed exceptional responses in terms of ORR (89%), PFS (HR: 0.17) and OS (HR: 0.24) for patients with POLE/POLD1 mutations compared to dMMR/MSI-H patients. For this reason, POLE/POLD1 mutations represent novel biomarkers predicting response to ICI.
Consensus Molecular Subtypes
The consensus molecular subtypes (CMS) for CRC, described in 2015, define four clusters based on molecular signatures [67]. Briefly, CMS1 (MSI immune) summarizes tumors with dMMR/MSI-H, high CIMP, hypermutation, BRAF mutation and immune infiltration/activation. CMS2 (canonical) shows WNT & MYC activation and chromosomal instability (somatic copy number alterations (SNCA). CMS3 (metabolic) harbour also dMMR/MSI-H, but with low CIMP and KRAS mutations. CMS4 (mesenchymal) tumors have a high SNCA burden, stromal activation, TGF-β-activation and promotion of angiogenesis [67].
The Checkmate 9X8 trial revealed that adding nivolumab to standard care in pMMR/MSS mCRC patients is able to extend PFS, particularly in CMS1 and CMS3 patients and those with KRASG12D/V/C mutations [38]. How KRAS mutations are involved in the tumor immune microenvironment and in the sensitivity/resistance of ICI is currently the focus of research. The REGONIVO trial investigated the efficacy of regorafenib plus nivolumab in mCRC showing higher PFS in the CMS4 subgroup [27]. Responders showed gene-set enrichments in epithelial-mesenchymal transition pathways. IHC analyses revealed that responders correlated with higher infiltrations of CD8+ T cells, Tregs and M2 macrophages, thus constituting a “hotter” tumor microenvironment [68].
Assessing the Tumor Microenvironment
ICI efficacy is heavily dependent on the activity of the patient’s immune system. It follows that the immediate tumor microenvironment is of interest for judging susceptibility to ICI, especially in the context of pMMR/MSS. Besides the AtezoTribe [37], the CAMILLA trial investigated cabozantinib with durvalumab in chemorefractory gastrointestinal cancer and showed similar findings using a transcriptomic approach. Gene-set variance analysis revealed increased interferon-γ signaling activity, IL-1 and IL-6 expression and T cell activity in patients who has shown a response to treatment. Moreover, differential gene expressions in non-responders and responders showing upregulation of genes associated with protein processing and changes in peptidase activity in responders, potentially affecting antigen presentation/processing [69]. These findings illustrate that characterizing the TME through different methods like DGE and subsequent GSVA could have potential impact on therapeutic choices, despite their limited availability and affordability.
Impact of Liver Metastases
One putative negative “biomarker” whose impact has been shown is the comparative lack of efficacy of ICI in patients with liver metastasis. In a trial by Chongkai Wang et al., the ORR was approximately 20% in patients without liver metastases versus approximately 2% in those with liver metastases. Median PFS was 4.0 vs. 1.5 months for patients without versus with liver metastasis. These data suggest that mCRC MSS patients with liver metastases may benefit less from ICI therapy [70]. Data from another trial confirmed this data [21]. The investigators could show higher responses in mCRC patients without liver metastases when treated with an ICI combination. However, more prospective data are clearly necessary to corroborate these findings.
Circulating Tumor DNA
Loree et al. used serial circulating tumor (ct)DNA to detect MAPK pathway alterations (SNV or indel in RAS/EGFR/BRAFV600 or MET or ERBB2 amplification) after various therapy lines. They could show that the exposure to anti-EGFR antibodies doubled the risk of new MAPK-alterations and increased the TMB, particularly in patients without existing MAPK alterations. By monitoring TMB with liquid biopsies, conclusions may be drawn about the efficacy of ICIs and patients who did not qualify initially might do so over the course of their treatment – i.e. making the tumor “hotter” by using anti-EGFR therapy. Applying this concept to monitor ICI using serial ctDNA might allow prediction of the development of secondary resistance mechanisms. Furthermore, anti-EGFR therapy induced TMB might contribute to making a tumor susceptible to ICI immunotherapy [71].
Gut Microbiota
Microbiota alter response to ICI in various cancer types. One of the first cancers in which ICI has revolutionized the therapeutic management was in melanoma [72]. Routy et al. found that antibiotic therapy had an impact on the efficacy of PD1 inhibitors [73] and Vopalakrishnan et al. [74] showed that the gut microbiome composition influences therapeutic response and that a relative abudance of Ruminococcaceae bacteria may be advantageous.
Further studies [75, 76] showed that Serratia, Cupriavidus and Sphingobium species are increased in pMMR cancer patient’s microbiota. An additional study proposed a microbiota-based classification of CRC showing three subgroups with differences in survival rate and MSI status [77]. Despite the interaction between microbiota, cancerogenesis and treatment response, therapeutic consequences remain limited. Pre-clinical trials in mice with MSS CRC [78] showed that altering the gut microbiome using antibiotics blunted the response to anti-PD1 inhibitors, while Lactobacillus adiophilus lysates enhanced the efficacy of anti-CTLA4 inhibitors [79]. Various pre-clinical trials have shown that altering the gut microbiota in mice showed effects on ICI efficacy [80, 81].
Cytokines
IL17A
IL17A, a cytokine, shows to be overexpressed in CRC [82] and has several effects on the TME. It induces the synthesis of PGEF2, which surpasses CXCL9/10 impeding infiltration of the TME by CD8+ T cells (see below.) [83]. Studies in mouse models of CRC [84] and cell lines [85] showed that IL-17 inhibits the recruitment of CD8+ T cells and increased PDL1 expression, thereby promoting resistance to anti-PD1 therapy. Therapeutic antibodies for blocking IL-17A have been developed for psoriasis and ankylosing spondylitis and one trial was registered combining secukinumab (anti-IL17) with camrelizumab (anti-PD1) therapy, but the current trial status is still unknown [86].
Chemokines
Chemokines, a subset of cytokines, interact with G-protein coupled receptors, inducing chemotaxis and contributing to extravasation [87]. Several chemokines can predict ICI response in CRC and other cancers. One chemokine axis involves CXCL9, -10 and -11 with a common downstream activation of CXCR3. Interferon gamma stimulates CXCL9 production [88] by monocytes, endothelial cells, fibroblasts and cancer cells leading to increased chemotaxis and infiltration of the TME [89]. CXCR3, the receptor of CXCL9/10/11 serves as a prognostic biomarker for CRC. Intratumoral activity of the CXCR3-chemokine system is necessary for anti-PD1 to work [90]. Meta-analysis has shown that high CXCL9 levels are associated with a good response to ICI in several cancers [91].
Conclusion
Some biomarkers have been proposed for determining therapeutic susceptibility upon ICI in pMMR/MSS nCRC patients. Many represent surrogate markers of immune activity or immune cell infiltration of the TME. Some biomarkers, such as TMB or POLE/POLD1 mutations, represent genotypes that are probably not inducible de-novo in pre-existing disease or that present distinct entities mimicking MSI, whereas some markers might be viable as a means of judging how successful efforts of making the TME “hot” have been.
Future Perspective
Despite recent advances in identifying novel biomarkers and potential combinations of targeted therapies with chemotherapy or radiotherapy in MSS/pMMR mCRC, a definitive solution has not yet been established. The most promising approach so far involves radiotherapy combined with chemotherapy-free regimens, as highlighted by the NEST-1 trial, which demonstrate a higher likelihood of response to ICI in localized MSS/pMMR CRC with a low disease burden. Additionally, emerging strategies involving CAR-T cells, tumor vaccines, novel immune checkpoint inhibitors and biomarker stratified trials hold the potential to shape the future of oncology in this field.
Data Availability
No datasets were generated or analysed during the current study.
References
Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–44.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Leowattana W, Leowattana P, Leowattana T. Systemic treatment for metastatic colorectal cancer. World J Gastroenterol. 2023;29(10):1569–88.
Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, et al. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22(9):998–1009.
Kuboki Y, Fakih M, Strickler J, Yaeger R, Masuishi T, Kim EJ, et al. Sotorasib with panitumumab in chemotherapy-refractory KRASG12C-mutated colorectal cancer: a phase 1b trial. Nat Med. 2024;30(1):265–70.
Van Cutsem E, Huijberts S, Grothey A, Yaeger R, Cuyle PJ, Elez E, et al. Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From the Phase III BEACON Colorectal Cancer Study. J Clin Oncol. 2019;37(17):1460–9.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20.
André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383(23):2207–18.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91.
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36(8):773–9.
Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40(2):161–70.
Andre T, Elez E, Van Cutsem E, Jensen LH, Bennouna J, Mendez G, et al. Nivolumab (NIVO) plus ipilimumab (IPI) vs chemotherapy (chemo) as first-line (1L) treatment for microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): First results of the CheckMate 8HW study. JCO. 2024;42(3_suppl):LBA768.
Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44(2):343–54.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849–61.
Makaremi S, Asadzadeh Z, Hemmat N, Baghbanzadeh A, Sgambato A, Ghorbaninezhad F, et al. Immune Checkpoint Inhibitors in Colorectal Cancer: Challenges and Future Prospects. Biomedicines. 2021;9(9):1075.
Ree AH, Šaltytė Benth J, Hamre HM, Kersten C, Hofsli E, Guren MG, et al. First-line oxaliplatin-based chemotherapy and nivolumab for metastatic microsatellite-stable colorectal cancer-the randomised METIMMOX trial. Br J Cancer. 2024;130(12):1921–8.
Centre Georges Francois Leclerc. Phase Ib/II Trial Evaluating the Safety, Tolerability and Immunological Activity of Durvalumab (MEDI4736) (Anti-PD-L1) Plus Tremelimumab (Anti-CTLA-4) Combined With FOLFOX in Patients With Metastatic Colorectal Cancer [Internet]. clinicaltrials.gov; 2023 [cited 2024 Jan 1]. Report No.: NCT03202758. Available from: https://clinicaltrials.gov/study/NCT03202758.
Thibaudin M, Fumet JD, Chibaudel B, Bennouna J, Borg C, Martin-Babau J, et al. First-line durvalumab and tremelimumab with chemotherapy in RAS-mutated metastatic colorectal cancer: a phase 1b/2 trial. Nat Med. 2023;29(8):2087–98.
Agenus Inc. A Phase 1 Study of AGEN1181, an Fc-Engineered Anti-CTLA-4 Monoclonal Antibody as Monotherapy and in Combination With AGEN2034 (Balstilimab), an Anti-PD-1 Monoclonal Antibody, in Subjects With Advanced Cancer [Internet]. clinicaltrials.gov; 2023 [cited 2024 Jan 1]. Report No.: NCT03860272. Available from: https://clinicaltrials.gov/study/NCT03860272.
Bullock AJ, Schlechter BL, Fakih MG, Tsimberidou AM, Grossman JE, Gordon MS, et al. Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: a phase 1 trial. Nat Med. 2024. https://doi.org/10.1038/s41591-024-03083-7.
Dienstmann R, Salazar R, Tabernero J. The evolution of our molecular understanding of colorectal cancer: what we are doing now, what the future holds, and how tumor profiling is just the beginning. Am Soc Clin Oncol Educ Book. 2014;34:91–9. https://doi.org/10.14694/EdBook_AM.2014.34.91.
City of Hope Medical Center. A Phase I Clinical Trial of Regorafenib, Nivolumab, and Ipilimumab in Advanced Chemotherapy Resistant Metastatic MSS Colorectal Cancer [Internet]. clinicaltrials.gov; 2024 [cited 2024 Jan 1]. Report No.: NCT04362839. Available from: https://clinicaltrials.gov/study/NCT04362839.
Fakih M, Sandhu J, Lim D, Li X, Li S, Wang C. Regorafenib, Ipilimumab, and Nivolumab for Patients With Microsatellite Stable Colorectal Cancer and Disease Progression With Prior Chemotherapy: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2023;9(5):627–34.
Shitara K. Regorafenib and Nivolumab Simultaneous Combination Therapy for Advanced and Metastatic Solid Tumors: Phase I Clinical Trial [Internet]. clinicaltrials.gov; 2021 [cited 2024 Jan 1]. Report No.: NCT03406871. Available from: https://clinicaltrials.gov/study/NCT03406871.
Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023;58:101917.
Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61.
Qiu M. A Phase I Study on Tolerance and Safety of Vemurafenib Film-coated Tablets, Cetuximab Solution for Infusion and Camrelizumab Protocol(VCC) in the After Line Therapy of BRAF V600E Mutation/MSS Metastatic Colorectal Cancer [Internet]. clinicaltrials.gov; 2022 [cited 2024 Jan 1]. Report No.: NCT05019534. Available from: https://clinicaltrials.gov/study/NCT05019534.
Qiu M, Wei G, Leng W, Cao P. 624P Tolerability and safety of vemurafenib, cetuximab combined with camrelizumab for BRAF V600E-mutated /MSS metastatic colorectal cancer. Ann Oncol. 2023;34:S446.
Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44(3):609–21.
Exelixis. A Randomized Open-Label Phase 3 Study of XL092 + Atezolizumab vs Regorafenib in Subjects With Metastatic Colorectal Cancer [Internet]. clinicaltrials.gov; 2024 [cited 2024 Jan 1]. Report No.: NCT05425940. Available from: https://clinicaltrials.gov/study/NCT05425940.
Saeed A, Tabernero J, Wang G, Ma X, Smith R, Hecht JR. Zanzalintinib (XL092) plus atezolizumab versus regorafenib in previously treated MSS/MSI-low metastatic colorectal cancer (mCRC): The randomized phase 3 STELLAR-303 study. JCO. 2024;42(3_suppl):TPS22.
Kawazoe A, Xu RH, García-Alfonso P, Passhak M, Teng HW, Shergill A, et al. LEAP-017 investigators. Lenvatinib plus pembrolizumab versus standard of care for previously treated metastatic colorectal cancer: final analysis of the randomized, open-label, Phase III LEAP-017 Study. J Clin Oncol. 2024;JCO2302736. https://doi.org/10.1200/JCO.23.02736.
Bordonaro R, Calvo A, Auriemma A, Hollebecque A, Rubovszky G, Saunders MP, et al. Trifluridine/tipiracil in combination with oxaliplatin and either bevacizumab or nivolumab in metastatic colorectal cancer: a dose-expansion, phase I study. ESMO Open. 2021;6(5):100270.
Patel MR, Falchook GS, Hamada K, Makris L, Bendell JC. A phase 2 trial of trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable metastatic colorectal cancer. Cancer Med. 2021;10(4):1183–90.
Mettu NB, Ou FS, Zemla TJ, Halfdanarson TR, Lenz HJ, Breakstone RA, et al. Assessment of Capecitabine and Bevacizumab With or Without Atezolizumab for the Treatment of Refractory Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Netw Open. 2022;5(2):e2149040.
Antoniotti C, Rossini D, Pietrantonio F, Catteau A, Salvatore L, Lonardi S, et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23(7):876–87.
Lenz HJ, Parikh A, Spigel DR, Cohn AL, Yoshino T, Kochenderfer M, et al. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: phase 2 results from the CheckMate 9X8 randomized clinical trial. J Immunother Cancer. 2024;12(3):e008409.
Wainberg ZA, Han SW, Lee S, Lee KW, Kopetz S, Mizrahi J, et al. ARC-9: A randomized study to evaluate etrumadenant based treatment combinations in previously treated metastatic colorectal cancer (mCRC). JCO. 2024;42(16_suppl):3508.
Woolston A, Khan K, Spain G, Barber LJ, Griffiths B, Gonzalez-Exposito R, et al. Genomic and Transcriptomic Determinants of Therapy Resistance and Immune Landscape Evolution during Anti-EGFR Treatment in Colorectal Cancer. Cancer Cell. 2019;36(1):35-50.e9.
Gruppo Oncologico del Nord-Ovest. Phase II Study of AVELUMAB and CETUXIMAB and Modified FOLFOXIRI as Initial Therapy for RAS Wild-type Unresectable Metastatic Colorectal Cancer Patients [Internet]. clinicaltrials.gov; 2023 [cited 2024 Jan 1]. Report No.: NCT04513951. Available from: https://clinicaltrials.gov/study/NCT04513951.
Conca V, Antoniotti C, Bergamo F, Pietrantonio F, Rossini D, Scartozzi M, et al. Modified FOLFOXIRI plus cetuximab and avelumab as initial therapy in RAS wild-type unresectable metastatic colorectal cancer: Results of the phase II AVETRIC trial by GONO. JCO. 2023;41(16_suppl):3575.
Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57(8):1115–24.
Tapia Rico G, Price TJ. Atezolizumab for the treatment of colorectal cancer: the latest evidence and clinical potential. Expert Opin Biol Ther. 2018;18(4):449–57.
Gruppo Oncologico del Nord-Ovest. Randomized Phase II Study of FOLFOXIRI Plus Bevacizumab Plus Atezolizumab Versus FOLFOXIRI Plus Bevacizumab as First-line Treatment of Unresectable Metastatic Colorectal Cancer Patients. [Internet]. clinicaltrials.gov; 2023 [cited 2024 Jan 1]. Report No.: NCT03721653. Available from: https://clinicaltrials.gov/study/NCT03721653.
Moretto R, Rossini D, Catteau A, Antoniotti C, Giordano M, Boccaccino A, et al. Dissecting tumor lymphocyte infiltration to predict benefit from immune-checkpoint inhibitors in metastatic colorectal cancer: lessons from the AtezoT RIBE study. J Immunother Cancer. 2023;11(4):e006633.
Rückert M, Flohr AS, Hecht M, Gaipl US. Radiotherapy and the immune system: More than just immune suppression. Stem Cells. 2021;39(9):1155–65.
Yuan X. A Phase II, Single-arm, Open-label, Single Center Study of Tislelizumab Combined With Fruquintinib and SBRT as A Third-line and Posterior Line Treatment in Patients With Advanced Colorectal Cancer [Internet]. clinicaltrials.gov; 2022 [cited 2024 Jan 1]. Report No.: NCT04924179. Available from: https://clinicaltrials.gov/study/NCT04924179.
Yuan X, Zhang M, Hou H. Fruquintinib combined with tislelizumab and SBRT as a later-line therapy in microsatellite stability (MSS) metastatic colorectal cancer (mCRC): Results from the FRUIT trial. JCO. 2023;41(4_suppl):150.
Kasi PM, Jafari MD, Yeo H, Lowenfeld L, Khan U, Nguyen A, et al. Neoadjuvant botensilimab plus balstilimab in resectable mismatch repair proficient and deficient colorectal cancer: NEST-1 clinical trial. JCO. 2024;42(3_suppl):117.
Agenus Inc. A Randomized, Open-Label, Phase 2 Study of Botensilimab (AGEN1181) as Monotherapy and in Combination With Balstilimab (AGEN2034) or Investigator’s Choice Standard of Care (Regorafenib or Trifluridine and Tipiracil) for the Treatment of Refractory Metastatic Colorectal Cancer [Internet]. clinicaltrials.gov; 2024 [cited 2024 Jan 1]. Report No.: NCT05608044. Available from: https://clinicaltrials.gov/study/NCT05608044.
Forni G. Vaccines for tumor prevention: a pipe dream? J Infect Dev Ctries. 2015;9(6):600–8.
Kothari N, Postwala H, Pandya A, Shah A, Shah Y, Chorawala MR. Establishing the applicability of cancer vaccines in combination with chemotherapeutic entities: current aspect and achievable prospects. Med Oncol. 2023;40(5):135.
Berntsson J, Eberhard J, Nodin B, Leandersson K, Larsson AH, Jirström K. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: Relationship with sidedness and prognosis. Oncoimmunology. 2018;7(8):e1465165.
Wang L, Ren F, Wang Q, Baldridge LA, Monn MF, Fisher KW, et al. Significance of Programmed Death Ligand 1 (PD-L1) Immunohistochemical Expression in Colorectal Cancer. Mol Diagn Ther. 2016;20(2):175–81.
Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55.
Muquith M, Espinoza M, Elliott A, Xiu J, Seeber A, El-Deiry W, et al. Tissue-specific thresholds of mutation burden associated with anti-PD-1/L1 therapy benefit and prognosis in microsatellite-stable cancers. Nat Cancer. 2024. https://doi.org/10.1038/s43018-024-00752-x.
Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30(7):1096–103.
Duvivier HL, Rothe M, Mangat PK, Garrett-Mayer E, Ahn ER, Al Baghdadi T, et al. Pembrolizumab in Patients With Tumors With High Tumor Mutational Burden: Results From the Targeted Agent and Profiling Utilization Registry Study. J Clin Oncol. 2023;41(33):5140–50.
Merino DM, McShane LM, Fabrizio D, Funari V, Chen SJ, White JR, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8(1):e000147.
Marcus L, Fashoyin-Aje LA, Donoghue M, Yuan M, Rodriguez L, Gallagher PS, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin Cancer Res. 2021;27(17):4685–9.
Garmezy B, Gheeya J, Lin HY, Huang Y, Kim T, Jiang X, et al. Clinical and Molecular Characterization of POLE Mutations as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors in Advanced Cancers. JCO Precis Oncol. 2022;6:e2100267.
Domingo E, Freeman-Mills L, Rayner E, Glaire M, Briggs S, Vermeulen L, et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: a retrospective, pooled biomarker study. Lancet Gastroenterol Hepatol. 2016;1(3):207–16.
Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7.
Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu ZX, et al. Evaluation of POLE and POLD1 Mutations as Biomarkers for Immunotherapy Outcomes Across Multiple Cancer Types. JAMA Oncol. 2019;5(10):1504–6.
Ambrosini M, Rousseau B, Manca P, Artz O, Marabelle A, André T, et al. Immune checkpoint inhibitors for POLE or POLD1 proofreading-deficient metastatic colorectal cancer. Ann Oncol. 2024;S0923–7534(24):00104–12.
Guinney J, Dienstmann R, Wang X, De Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6.
Takei S, Tanaka Y, Lin YT, Koyama S, Fukuoka S, Hara H, et al. Multiomic molecular characterization of the response to combination immunotherapy in MSS/pMMR metastatic colorectal cancer. J Immunother Cancer. 2024;12(2):e008210.
Saeed A, Park R, Pathak H, Al-Bzour AN, Dai J, Phadnis M, et al. Clinical and biomarker results from a phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort. Nat Commun. 2024;15(1):1533.
Wang C, Sandhu J, Ouyang C, Ye J, Lee PP, Fakih M. Clinical Response to Immunotherapy Targeting Programmed Cell Death Receptor 1/Programmed Cell Death Ligand 1 in Patients With Treatment-Resistant Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. JAMA Netw Open. 2021;4(8):e2118416.
Loree JM, Bubie A, Eluri M, Parseghian CM, Overman MJ, Zhang N, et al. Clinical utility of serial circulating tumor DNA (ctDNA) to identify acquired resistance to anti-EGFR antibodies in metastatic colorectal cancer (mCRC). JCO. 2024;42(3_suppl):158.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Burns MB, Montassier E, Abrahante J, Priya S, Niccum DE, Khoruts A, et al. Colorectal cancer mutational profiles correlate with defined microbial communities in the tumor microenvironment. PLoS Genet. 2018;14(6):e1007376.
Jin M, Wu J, Shi L, Zhou B, Shang F, Chang X, et al. Gut microbiota distinct between colorectal cancers with deficient and proficient mismatch repair: A study of 230 CRC patients. Front Microbiol. 2022;13:993285.
Mouradov D, Greenfield P, Li S, In EJ, Storey C, Sakthianandeswaren A, et al. Oncomicrobial Community Profiling Identifies Clinicomolecular and Prognostic Subtypes of Colorectal Cancer. Gastroenterology. 2023;165(1):104–20.
Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, et al. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front Microbiol. 2020;11:814.
Zhuo Q, Yu B, Zhou J, Zhang J, Zhang R, Xie J, et al. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci Rep. 2019;9(1):20128.
Jia D, Wang Q, Qi Y, Jiang Y, He J, Lin Y, et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell. 2024;187(7):1651-1665.e21.
Li W, Zhou Q, Lv B, Li N, Bian X, Chen L, et al. Ganoderma lucidum Polysaccharide Supplementation Significantly Activates T-Cell-Mediated Antitumor Immunity and Enhances Anti-PD-1 Immunotherapy Efficacy in Colorectal Cancer. J Agric Food Chem. 2024;72(21):12072–82.
Sui G, Qiu Y, Yu H, Kong Q, Zhen B. Interleukin-17 promotes the development of cisplatin resistance in colorectal cancer. Oncol Lett. 2019;17(1):944–50.
Song M, Liang J, Wang L, Li W, Jiang S, Xu S, et al. IL-17A functions and the therapeutic use of IL-17A and IL-17RA targeted antibodies for cancer treatment. Int Immunopharmacol. 2023;123:110757.
Chen J, Ye X, Pitmon E, Lu M, Wan J, Jellison ER, et al. IL-17 inhibits CXCL9/10-mediated recruitment of CD8+ cytotoxic T cells and regulatory T cells to colorectal tumors. J Immunother Cancer. 2019;7(1):324.
Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. 2021;9(1):e001895.
Zhang Y. Phase Ib/IIa Study of SHR-1210 Combined With AIN457 for Patients With Late Stage MSS CRC Who Failed Second-line and Above Treatment [Internet]. clinicaltrials.gov; 2020 [cited 2024 Jan 1]. Report No.: NCT04298320. Available from: https://clinicaltrials.gov/study/NCT04298320.
Franciszkiewicz K, Boissonnas A, Boutet M, Combadière C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012;72(24):6325–32.
Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997;272(23):14899–907.
Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–7.
Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, et al. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity. 2019;50(6):1498-1512.e5.
Litchfield K, Reading JL, Puttick C, Thakkar K, Abbosh C, Bentham R, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184(3):596-614.e14.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Contributions
All authors contributed in the preparation of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamid, M.A., Pammer, L.M., Lentner, T.K. et al. Immunotherapy for Microsatellite-Stable Metastatic Colorectal Cancer: Can we close the Gap between Potential and Practice?. Curr Oncol Rep (2024). https://doi.org/10.1007/s11912-024-01583-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-024-01583-w