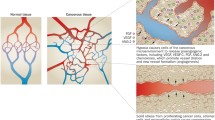
Abstract
Purpose of the Review
Angiogenesis plays a key role in bladder cancer (BC) pathogenesis. In the last two decades, an increasing number of publications depicting a multitude of novel angiogenic molecules and pathways have emerged. The growing complexity necessitates an evaluation of the breadth of current knowledge to highlight key findings and guide future research.
Recent Findings
Angiogenesis is a dynamic biologic process that is inherently difficult to assess. Clinical assessment of angiogenesis in BCs is advancing with the integration of image analysis systems and dynamic contrast-enhanced and magnetic resonance imaging (DCE-MRI). Tumour-associated macrophages (TAMs) significantly influence the angiogenic process, and further research is needed to assess their potential as therapeutic targets. A rapidly growing list of non-coding RNAs affect angiogenesis in BCs, partly through modulation of vascular endothelial growth factor (VEGF) activity. Vascular mimicry (VM) has been repeatedly associated with increased tumour aggressiveness in BCs. Standardised assays are needed for appropriate identification and quantification of VM channels.
Summary
This article demonstrates the dynamic and complex nature of the angiogenic process and asserts the need for further studies to deepen our understanding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is the tenth most common malignancy globally and the sixth most common cancer in men [1]. Most BCs originate from the urothelium, and the most common type is known as urothelial carcinoma or transitional cell carcinoma. Less common types include adenocarcinoma and squamous cell carcinoma [2].
Approximately, 70% of urothelial carcinomas present as superficial cancers confined to the mucosa with a high recurrence rate following transurethral resection. About a third of non-muscle invasive cancers will progress to higher grade more invasive tumours. Muscle-invasive carcinomas are usually associated with metastatic disease and carry a poor prognosis [3]
Angiogenesis is a key step in tumour survival and progression in BCs. Vascular endothelial growth factor (VEGF) has been widely investigated due to its vital role in BC angiogenesis, and its expression has been mostly associated with worse outcomes [3, 4]. Studies investigating angiogenesis in BCs are expanding, and current knowledge projects an increasingly complex picture supporting the need for better understanding of the biologic concepts of angiogenesis to improve disease characterisation and patient-targeted therapeutic strategies.
In this article, we discuss the methods employed in the clinical assessment of angiogenesis in BCs and highlight existing limitations. Of particular interest, we highlight the various angiogenic molecules that have emerged in the past two decades. We discuss the role of the tumour microenvironment and the impact of inflammation and TAMs on the angiogenic process. We shed light on alternate regulatory mechanisms such as non-coding RNAs. We finally bring to attention unconventional forms of angiogenesis as we discuss VM and its significance in BCs.
Clinical Assessment of Angiogenesis in Bladder Cancers
Angiogenic activity in BCs has been long employed as a parameter for the characterisation and evaluation of tumour behaviour and aggressiveness. Assessment of angiogenesis is understandably a complex process owing to its innate dynamic nature. Micro-vessel density (MVD), as the end product of the angiogenic process, has been the most used method to assess angiogenic activity in BCs. Various angiogenic molecules as drivers of the angiogenic process have been evaluated for potential use as predictors of prognosis. Alternatively, imaging techniques have been shown to be the most valuable tools in assessing vascularity in clinical trials offering both serial and spatial assessment of the process.
Micro-Vessel Density
MVD has long been used as a prognostic parameter in many cancers. In 1991, Weidner et al. introduced the idea of MVD for the assessment of tumour angiogenesis. MVD describes the number of blood vessels within a defined number of microscopic fields “hotspots” [5]. In BCs, MVD has been correlated with various clinical aspects such as increased mortality and recurrence [6,7,8]. MVD has also been associated with the expression of various biological markers including p53, VEGF-C, TSP-1, AT1R, Maspin, nitric oxide, and DNA ploidy, to name a few [4, 9,10,11].
The hotspot technique is mostly used to quantify intra-tumoural blood vessels identified by pan-endothelial cell markers such as CD34, CD31, or von Willebrand factor (vWF). Hotspots are identified by light microscopy, and individual micro-vessels are counted at high magnification in the identified spots [6, 7]. A systematic review in 2014 has shown that high MVD was significantly associated with poor survival [8]. Despite general agreement across the literature, the technique has not yet reached the level of evidence for widespread routine clinical utilisation. Inter-observer variability of vessel counting remains a significant barrier requiring the application of strict counting rules and objective training of individual observers. The diverse nature of studied samples (transurethral resections vs cystectomy specimens) and the likelihood of unrepresentative sampling of micro-vessel hotspots in fragile papillary tumours also contribute to the limited utilisation of MVD as a prognostic parameter [12••].
Intra-tumoural MVD represents the collective interplay between pre-existing vasculature and neovascularisation. Double immunostaining with endothelial cell-specific antibodies such as CD34 or Factor VIII and cycling nuclei-specific antibodies such as Ki67 or proliferating cell nuclear antigen (PCNA) had been employed to highlight ongoing angiogenesis [13].
Novel markers offering the ability to quantitatively distinguish between tumour neovascularisation and pre-existing vessels have been identified. Notably, endoglin (CD105), an accessory receptor for transforming growth factor beta (TGF-β), has been shown to be overexpressed in vascular endothelial cells of tissues undergoing active angiogenesis such as regenerating and inflamed tissues or tumours [14]. Furthermore, levels of endoglin/CD105 have been shown to positively correlate with the extent of endothelial cell proliferation and with the expression of proliferation markers in tumour endothelia [15,16,17]. Intra-tumour micro-vessel density assessed by endoglin/CD105 staining has been shown to strongly correlate with prognosis in many cancer patients [15,16,17,18,19].
Miyata et al. compared the performance of CD31, CD34, and CD105 in a series of urothelial cancers. CD105 MVD showed the strongest association with stage, grade, and VEGF-A expression [20]. Agrawal et al. demonstrated an association between VEGF, CD105, and p21 expression in 90 cases of non-muscle invasive bladder cancers (NMIBCs) where combination profiles of the three markers significantly predicted hazard for recurrence [21]. A final study investigated the expression of CD105 in 50 biopsies of urinary bladder carcinomas and 15 benign bladder biopsies from Iraqi patients and concluded that CD105 expression was significantly associated with grade in urinary bladder carcinomas [22].
A recent study projects endoglin as a crucial molecule in the determination of an immunosuppressive tumour microenvironment, mainly because of its role on angiogenesis but also in inflammation and in cancer-associated fibroblast (CAFs) biology. Endoglin expression in patient biopsies could be an excellent biomarker of the immunosuppressive tumour microenvironment and may predict patients’ response to immunotherapy [23•]. Considering the limited number of published studies, the potential of endoglin as a therapeutic target and prognostic marker remains to be under-investigated in BCs.
MVD counts represent the combined product of all angiogenic pathways and as such are useful indicators of the effectiveness of anti-angiogenic therapy. Automation of counting methods with the use of image analysis systems promises increased speed and reliability. The development of algorithms for quantification of blood vessels supported by a user-friendly graphical interface can facilitate histopathological assessment and increase reproducibility [24••]. The requirement of obtaining serial biopsy samples during treatment is, however, invasive and subject to inaccuracy due to regional heterogeneity within individual tumours [25].
Key (Conventional) Angiogenic Molecules
Evolving neovascularisation in tumours is the result of complex dynamic processes involving an imbalance between pro- and anti-angiogenic factors. The main signalling molecules of vascular morphogenesis include VEGF, angiopoietins (ANGPTs), ephrins, TGF-β, and platelet-derived growth factor (PDGF). The anti-angiogenic factors include angiostatin, endostatin, interferon, platelet factor 4, thrombospondin, prolactin 16 kD fragment, and tissue inhibitors of metalloproteinase-1, 2, and 3 [26].
Among the many studied angiogenic factors, VEGF plays a significant and principal role in BC angiogenesis. Elevated levels of VEGF expression are generally associated with worse outcomes for patients with BC [4, 27,28,29,30,31, 32•]. Notably, the level of VEGF expression did not predict outcomes for patients with BC in many studies [33,34,35,36,37]. A meta-analysis including 11 studies showed that elevated levels of VEGF are associated with poor prognosis in patients with BC [37]. The authors, however, highlight several important limitations in interpreting their studies of which heterogeneity emerged as a key factor. Immunohistochemistry, ELISA (enzyme-linked immunosorbent assay), Western blot, and RT-PCR are among the commonly employed detection techniques. Various anti-VEGF antibodies (VEGF A, C, and D) are used. Variability of studied samples is also noted (urine, serum, and tissue samples). Collectively, these factors contribute to the difficult interpretation of the studies [37].
Other markers including basic fibroblast growth factor (bFGF), thrombospondin 1, HIF 1, 2, and 3, interleukin–8 (IL-8), cyclooxygenase-2 (COX-2), and matrix metalloproteinases (MMPs) have also showed promising results in studies correlating these markers with various clinicopathologic parameters [38,39,40]. A systematic review in 2017 examining the prognostic role of MMPs in BCs indicated that high expression of MMPs was significantly associated with poor prognosis [41]. The authors advice cautious interpretation of the results owing to limitations of the number of included studies reporting on survival parameters, the variability of assays among the studies as well as the use of median/mean values as cut-off point.
Imaging of Angiogenesis in Bladder Cancers
Several imaging techniques have been developed to analyse tumour vasculature including computerised tomography (CT), positron emission tomography (PET), and ultrasonography. These techniques offer the advantage of serially monitoring spatially localised changes in tumour microcirculations [25]. Dynamic contrast-enhanced and magnetic resonance imaging, dual-energy CT (DECT), and contrast-enhanced ultrasound (CEUS) are among the techniques employed in investigating patients with BC. Of these, MRI is commonly employed in clinical trials of genitourinary tumours [25].
Precise identification of regional angiogenic activity in tumours is a potentially useful tool for guiding treatment selection and monitoring response. Angiogenic imaging poses a significant potential in improving diagnosis, staging, and response monitoring in bladder tumours as evidenced in many clinical trials [25]. DCE-MRI has been shown to be a useful technique able to distinguish leaky, disorganised tumour neo-vessels from mature well-organised vasculature. Studies have demonstrated a positive correlation between DCI-MRI parameters and MVD, histological grade, and stage [25, 42, 43]. The role of angiogenesis imaging in monitoring BC response to treatment has been evaluated in some studies. A study reported that DCE-MRI was significantly more accurate than conventional MRI in predicting a lack of response following 4 cycles of chemotherapy [44]. Another study suggests that DCE-MRI might be a reliable tool in excluding the presence of persistent or recurrent tumours up to 12 months after radiotherapy [45].
Superb microvascular imaging (SMI), an emerging Doppler ultrasound technique, employs an algorithm that has been shown to effectively assess micro-vessels and their distribution. The technique provides valuable information for diseases associated with angiogenesis than other non-invasive techniques. A few studies have demonstrated the effectiveness of SMI in assessing thyroid nodules, breast tumours, and lymph node diseases [46•]. A case report has shown that SMI helped in the detection of a bladder neoplasm avoiding additional cross-sectional imaging [47]. Further studies are needed to assess the role of SMI in monitoring treatment response in BC patients.
Alternate Factors Influencing Angiogenesis in Bladder Cancers
Decades of research into angiogenic molecules in BCs have unravelled a multitude of potential angiogenic factors and molecules. Functional relation to angiogenesis is demonstrated through association with various classic angiogenic parameters such as MVD and VEGF expression in many of the studies (Table 1). The studies demonstrate the complexity of the regulatory pathways influencing the angiogenic process. Further research is needed to characterise the clinical usefulness and biological significance of individual markers.
The Regulatory Role of Tumour Microenvironment and Its Impact on Bladder Cancer Angiogenesis
Non-Coding RNAs and Bladder Cancer Angiogenesis:
MicroRNAs
The role of miRNAs has been rigorously investigated since 2006 to identify their molecular networks and target genes in BCs. Several reviews have been published throughout the past few years depicting the significance of miRNAs as potential markers for BC screening and prognostication [79,80,81, 82••, 83, 84, 85•, 86,87,88, 89•, 90••]. A few studies have investigated the role of miRNAs in BC angiogenesis (Table 2).
Long non-coding RNAs
Long non-coding RNAs (lncRNAs) are non-protein coding RNAs with more than 200 nucleotides in length with the ability to down/upregulate gene expression [101,102,103]. There are more than 100 dysregulated lncRNAs in BC [104••]. LncRNAs exhibit tumour-suppressor and tumour-promoting roles, tightly regulating apoptosis, glycolysis, and EMT in BC. LncRNAs regulate immune cell infiltration in the tumour microenvironment and affect the response of BC cells to immunotherapy. lncRNAs are also able to regulate microRNAs, STAT3, Wnt, PTEN, and PI3K/Akt pathways affecting both the proliferation and migration of BC cells [105••, 106••]. Several studies have investigated the role of lncRNAs in BC angiogenesis (Table 3).
Circular RNAs
Hundreds of circular RNAs (circRNAs) are significantly dysregulated in human BC tissues [114]. Most circRNAs regulate BC through miRNA sponging regulatory mechanisms. Most have been reported to be associated with many clinicopathologic characteristics of BC, including tumour size, grade, differentiation, and stage; lymph node metastasis; tumour numbers; distant metastasis; invasion; and recurrence [115••]. Studies investigating the role of circRNAs in BC angiogenesis are presented in (Table 4).
Metabolic Derangements and Angiogenesis in Bladder Cancer
Recent studies demonstrate a link between abnormal metabolic processes and uncontrolled angiogenesis in BCs. In one study, the role of small extracellular vesicles in reprogramming glucose metabolism by increasing hexosamine biosynthesis pathway flux in endothelial cells in response to glutamine fructose-6-phosphate aminotransferase (GFAT) is demonstrated suggesting that inhibiting small extracellular vesicle-mediated GFAT1 secretion from BC cells may serve as novel anti-angiogenetic therapy [121•]. Hepatitis B X-interacting protein (HBXIP), a marker associated with a poor prognosis for BC, was shown to reduce glycolysis in BC cells via regulation of AKT/mTOR signalling, thereby blocking BC angiogenesis. This study provides a potential strategy to target HBXIP and AKT/mTOR for regulating glycolysis progression concurrently with anti-angiogenesis effects [122].
Cholesterol metabolism plays a significant role during cancer progression. The farnesoid X receptor (FXR) is a bile acid-activated transcription factor and a member of the nuclear receptor superfamily. Two studies have shown that FXR contributes to BC cell migration, invasion, and angiogenesis through the proteasomal degradation pathway. FXR overexpression induces AMPK phosphorylation and decreases cholesterol synthase-related protein expression. Statin usage showed potent enough efficacy to strengthen the enhancement of FXR-inhibited migration, adhesion, and angiogenesis in human urothelial carcinoma cells [123, 124].
Immunologic Factors: Relation to Angiogenesis in Bladder Cancers
BCs are considered as highly immunogenic malignancies capable of manipulating the host immune system to facilitate the survival and progression of their cancerous cells [125].
In BCs, both innate and adaptive immunity have been shown to contribute to carcinogenesis. TAMs, myeloid-derived suppressor cells, T cells, B cells, and their associated cytokines and chemokines have been shown to play a role in bladder carcinogenesis [126]. Of these, TAMs have been shown to directly contribute to angiogenesis in a few studies.
Tumour-Associated Macrophages
Multiple studies have shown that TAMs can produce multiple angiogenic factors including VEGF, TNF-α, IL-1β, IL-8 (CXCL8), PDGF, bFGF, thymidine phosphorylase, and MMPs [127]. Several studies have investigated the relationship between BCs and TAMs including their effect on BCG (Bacillus Calmette Guerin) treatment in in situ carcinomas, prognostic significance, the mechanisms involved in their activation and polarisation and their role in angiogenesis [126]. Hanada et al. assessed TAMs and micro-vessel counts in 63 patients with BC. Immunohistochemistry using anti-CD34 antibody was used to identify micro-vessels, and anti-CD-68 antibody was used to identify TAMs in tumour tissues. The results showed that TAMs and micro-vessel counts values in invasive BCs were significantly higher than in superficial tumours. There was also a positive correlation between TAM count and micro-vessel count in this study suggesting a prognostic role of TAM count in BC [128].
In 2016, Takeuchi et al. investigated TAM and micro-vessel counts in 17 NMIBC and 4 invasive cases. M2 macrophages were identified by immunohistochemical staining with anti-CD-68 and anti-CD163 antibodies identifying both the macrophage lineage and an M2-specific surface receptor, respectively. Micro-vessel counts were determined using immunohistochemistry with anti-CD34. Their results showed that the higher ratio of CD163+/CD68+ macrophages in the stroma, tumour, and total tumour tissues was significantly correlated with a higher stage and grade. In addition, the low ratio of CD68+/CD34+ micro-vessels was significantly correlated with a higher stage. There was also a positive correlation between TAMs and micro-vessel counts [129]. Furthermore, a study investigating the role of CXCL8 secreted by TAMs in urothelial carcinoma showed the infiltration of TAMs in the tumour microenvironment led to the elevation of CXCL8, which in turn promoted the secretion of MMP-9, VEGF, and E-cadherin by BC cells. CXCL8 altered the migration, invasion, and pro-angiogenic capacity of BC cells and accelerated cancer progression [130].
Chronic Inflammation and Angiogenesis in Bladder Cancer
BC is a chronic inflammation-associated type of neoplasia. To this effect, studies have shown that chronic inflammation whether local or systemic increases the risk of developing BCs [131]. BC cells have been shown to secrete various pro-inflammatory molecules that contribute to their advancement through increasing proliferation, angiogenesis, invasion, and metastasis [132].
Several signalling pathways have been linked to the initiation and progression of BCs during inflammation, including COX-2/nitric oxide synthase (NOS), janus activated kinase (JAK)-STAT3, the nuclear factor-kappaB (NF-κB), and PI3K-Akt-mTOR [131]. Nitric oxide generation from inducible isoform of nitric oxide synthases in the malignant epithelium and from endothelial isoform in tumour stroma has an important potential in the angiogenesis of BC [10]. NF-κB activation also mediates angiogenesis and metastasis in BC through the regulation of IL-8 [133].
Several pro-inflammatory cytokines have been associated with BC pathogenesis, but a few have been linked to angiogenic activity in these tumours. IL-6 was found to promote angiogenesis and vascular modelling via VEGF and STAT3, which affects the genes mediating angiogenesis. IL-6 silencing vector attenuated angiogenesis as demonstrated by the staining of CD31 and VEGF [134]. TNF-α also promotes angiogenesis and the development of several tumour types. In a study by Feng et al., expressional changes of Pigment epithelium-derived factor (PEDF) and TNF-α were related to angiogenesis of bladder tumours. TNF-α expression was positively correlated with MVD, while PEDF was negatively correlated with MVD [135]. Studies have demonstrated that IL-8 expression enhances angiogenic activity through the induction of MMP9 and subsequently regulates the tumourigenesis and production of spontaneous metastases in BCs [136]. The levels of human neutrophil peptide (HNP)-1, -2, and -3, produced by neutrophils, were found to be increased in BC with an effect on tumour angiogenesis and growth. All three HNPs are subtypes of α-defensins, proteins that aid in the recruitment of leukocytes. The indirect effects of HNPs 1–3 include stimulation of tumour cell proliferation and potentially tumour angiogenesis [137].
CD74 and macrophage migration inhibitory factor (MIF) were found to be expressed in MIBC samples, and only one high-grade BC cell line, HT-1376, compared with normal, NMIBC samples. The tumourigenesis and MVD assays indicated less proliferation and angiogenesis in the knockdown-HT-1376 cells [138]. Angiogenin (ANG), a member of the RNase A superfamily, has been demonstrated to promote tumour angiogenesis and metastasis in BC by activating key downstream target molecules of the PI3K-AKT-mTOR signalling pathway [139]. The urinary levels of ANG and angiostatin and the marker of oxidative stress, 8-iso-prostaglandin F2α (8-iso-PGF2α), the tumour progression marker ɣ-synuclein as well as IL-13 were shown to increase with the development of BC. These results further strengthen the interactive relationship between angiogenesis, oxidative stress, and inflammation in the pathogenesis and development of BC [140, 141]
Vascular Mimicry as a Distinct Angiogenic Mechanism in Bladder Cancers
VM was originally described in melanomas [142]. The term denotes the formation of microcirculatory systems within tumour tissues that do not require the presence of endothelial cells. VM vessels are instead formed by tumour cells creating channels that connect directly with the normal surrounding blood vessels to deliver erythrocytes and nutrients to tumourous tissues [143]. The cellular and molecular events underlying the formation of VM remain unclear despite many studies investigating factors related to cell migration, invasion, and matrix remodelling and their relation to VM formation [144].
In vivo Characterisation of Vascular Mimicry
In vivo characterisation of VM vessels was initially assessed by histological examination of tumour tissues/xenografts stained with CD34/CD31 combined with PAS staining where VM channels appear as tubule-like structures containing red blood cells. The tubules are positive for PAS staining and negative for CD34/CD31 contrasting with surrounding endothelium-dependant vessels which are positive for routine endothelial cell markers [142, 145]. These channels have also been shown to express elevated levels of genes associated with the multipotent, stem cell-like phenotype [146,147,148].
Based on PAS staining, VM vessels can present as straight channels, parallel straight channels, parallel straight channels with cross links, open arcs, arcs with branching, closed loops and networks. The vessels are matrix-rich structures, rich in laminin, proteoglycans, heparan sulphate, and collagens IV and VI as a part of their basement membranes visualised by the PAS staining. The tubular formations are sometimes referred to as “pattern structures” [149, 150].
Further studies into VM revealed more markers differentiating VM cells from normal endothelial cells including TIE-2, TIE-1, VEGFR-1 and 2, P-selectin, VCAM, CD106, Neuopilin, endoglin, and LAMC2, to name a few [151••]. Although the presence of PAS + tubular structures containing RBCs is accepted as an indicator of VM in the literature, it is believed that it cannot be independently used as a definitive proof of VM [152]. PAS + structures have been suggested to cross over true blood vessels containing RBCs, and cancer cells can secrete copious amounts of PAS + mucoproteins, but this should not necessarily imply vessel formation. Electron microscopy studies have not shown blood components inside VM channels in some studies, and many studies have failed to provide adequate imaging evidence of VM [153, 154]. These findings have raised scepticism about the methods used to identify VM in many studies in the literature [152].
There is currently no definitive VM marker that characterises non-endothelial vessels. More studies are needed to explore potential panels of biomarkers to improve the identification of VM vessels in vivo and in vitro.
In Vitro Characterisation of Vascular Mimicry
In vitro characterisation of VM was initially assessed on models using a 3D matrix (Matrigel) where the presence of intercellular connections was used as evidence for VM [155]. The concept was then reinforced by Francescone et al. characterising a Matrigel-based tube formation assay to assess VM in tumours. Ever since, this article has been cited as a reference for validating the use of intercellular connections as evidence of VM [156]. Notably, most of the studies investigating VM in vitro have utilised intercellular connections formed between cancer cells to report the presence and mechanisms of this phenomenon [152].
Valdivia et al. outline a strong argument that intercellular connections in an in vitro model do not necessarily represent fluid-containing vessels. In their article, the authors provide ample evidence based on a thorough literature review suggesting that most structures presented as VM in the literature may not in fact contain a lumen and thus cannot be regarded as fluid conducting vessels. The authors further highlight that only a few in vitro studies have persuasively demonstrated a functional lumen in tubular structures [157,158,159,160,161,162,163,164]. Building on these studies, the authors describe an in vitro model that utilises Matrigel to demonstrate tubular structures with microinjected trypan blue dye to illicit the movement of fluid. The use of confocal microscopy and IMARIS (Microscopy Image Analysis Software) reconstruction further confirms the presence of a lumen and a glycoprotein-rich layer flanked by cancer cells in their suggested model [152].
Vascular Mimicry in Bladder Cancer
The presence of VM in BCs has been investigated in a few studies. In these studies, VM was either assessed in vivo and/or in vitro using the classical methods described above.
In a study assessing the impact of VM on recurrence-free survival in urothelial carcinoma of the bladder, it was concluded that VM seemed to predict the risk of developing lung metastases after radical cystectomy. The combination of VM and TNM stage showed a better prognostic value than TNM stage alone or VM alone. The presence of VM also identified a subgroup of patients with MIBC who appeared to benefit from adjuvant chemotherapy. VM vessels in this study were identified using CD31-PAS double staining particularly if they contained RBCs [165].
In an earlier study, ECV304 human BC cells were used to determine how tumour cells take part in tumour neovascularisation. Subcutaneous ECV304 xenografts in mice showed various vessel types, including angiogenic vessels, tumour cell-related vessels, and extracellular matrix networks. ECV304 cells, cultured on collagen I gels, formed tube networks with the expression of several endothelial-related markers. The study concluded that ECV304 cells possess characteristics which confer the ability to mimic endothelial cells and facilitate the formation of VM. Vessels in this study were characterised using CD34, CD31, vWF, and Azan staining [166].
In a subsequent study, the cell line currently known as T24/83 was used to create a model of in vitro vasculogenic mimicry. In co-cultures of ECV304 and C378 human fibroblasts, tubular structures were identifiable after 8 days. The tubular structures showed elevated levels of transglutaminase 2 (TG2) antigen and TG2 in situ activity. In situ activity for TG2 showed co-localisation with both fibronectin and collagen IV. Deposition of these proteins into the extracellular matrix was reduced by the inclusion of non-cell penetrating TG inhibitors. Incubation of ECV304 cells with these same irreversible inhibitors reduced cell migration which paralleled a loss in focal adhesion assembly, actin cytoskeleton formation, and fibronectin deposition. The study concluded that TG2 appears to be essential for ECV304 tube formation, thus representing a potential novel therapeutic target in the inhibition of VM [167].
Yu et al. explored the expressions of CD133 and CD82/KAI1 in urothelial carcinoma and their relation to VM. Using immunohistochemistry, the positivity rates for these markers were significantly different between normal bladder epithelium and urothelial carcinomas where CD82 was downregulated in carcinomas and CD133 showed upregulation in cancerous tissues. Positive expressions of CD133, CD82/KAI1, and VM were significantly correlated with pTNM stage and tumour relapse but not with gender, age, or tumour numbers. Respectively, CD133 expression was positively correlated with VM, and CD82/KAI1 expression was negatively correlated with VM and CD133 [168].
In a study using BC cell lines UM-UC-3 and J82, and the immortalised human bladder epithelium cell line SV-HUC-1; 3-D cultures were constructed to detect VM formation. UM-UC-3 and J82 cells exhibited VM formation; however, SV-HUC-1 did not. Furthermore, VM-forming cancer cell lines UM-UC-3 and J82 exhibited higher zinc finger E-box binding homeobox 1 (ZEB1) expression. VM was observed in 31.1% of specimens from BC tissues, and cases with high ZEB1 expression accounted for 60% of patients. In addition, ZEB1 expression was significantly associated with VM and increased as the grade and stage of the tumour developed. The study concluded that ZEB1 may be associated with VM in BC and serve a key role in the process of VM formation [145].
Finally, androgen receptor was found to increase BC metastasis through activating VM formation. This was evidenced through altering the expression of the VM marker SLPI (secretory leukocyte protease inhibitor) through miR-525-5p which was decreased via binding to different androgen-response-elements located at distinct positions in the miR-525 precursor promoter [169].
The potential of VM as a therapeutic target in advanced high-grade BCs and in anti-angiogenic refractory patients remains to be investigated. The field of VM is currently marred with controversy demanding the development and standardisation of assays for the detection and quantification of VM in both in vitro and in vivo conditions. Additional studies are also needed to further characterise biomarkers/pathways of VM in BCs.
Perspectives and Concluding Notes
The studies summarised in this review undoubtedly illustrate the exponential evolution of current understanding of the tumour ecosystem. While VEGF, FGF, and PGF remain as the quintessential angiogenic molecules, the volume of suggested angiogenic regulators depicts a picture of increasing complexity and necessitates an integrative approach targeting angiogenesis at the structural, functional, and molecular levels. The tumour microenvironment holds the key for the future advancement of cancer detection and prognosis. While TAMs play a significant role in BC angiogenesis, the potential of reprogramming TAMs to induce vessel normalisation in BCs is yet to be fully investigated [170••].
There is a need for reliable and non-invasive biomarkers for assessing angiogenic activity in BCs. Current evidence strongly points to the role of non-coding RNAs in BC aetiopathogenesis. The abundance, conservation, and stability of non-coding RNAs render them potential effective diagnostic and prognostic biomarkers for BC. Analytical difficulties exist due to low expression levels of many non-coding RNAs and the bias introduced by RNA-seq library preparations [171•]. Exosomes are natural delivery vehicles for angiogenic and anti-angiogenic factors including non-coding RNAs. There is limited understanding of the biologic complexity of exosomes, and further proteomic analyses are needed to characterise their role in the tumour microenvironment. Notably, the shortage of standardised effective methods for exosome isolation, identification, and precise characterisation limits their application in clinical settings [172•].
Recently, extensive development of computational and in vitro experimental models to recapitulate tumour-endothelial cell interactions has posed the potential of a better understanding of the angiogenic process. Microfluidic chips have shown superior potentials for reflecting in vivo geometrical complexities, hydrodynamic stress, and mass transport [173, 174]. These models offer the possibility of creating microenvironments with regional heterogeneity and controllable and quantifiable spatiotemporal gradients [174, 175].
Computational models have accompanied experimental assays from the early days of research on tumour angiogenesis. In silico models of the tumour microenvironment have covered various aspects of tumour-stroma interactions. In contrast to microfluidic chips, in silico models embrace the complexity of a real tumour microenvironment avoiding the shortcomings of in vitro assays [174]. The integrated use of microfluidic models to validate mathematical models promises further opportunities to overcome the limitations inherent to both models. However, the scarcity of experimental and clinical data needed for the building of predictive experimental-theoretical platforms poses a challenge [174].
Ultimately, angiogenesis is a key biological event in BC carcinogenesis. Integration of angiogenic parameters within risk stratification tools will undoubtedly improve prognostication in BCs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; https://doi.org/10.3322/caac.21660 .
Afonso J, Freitas R, Lobo F, Morais A, Oliveira J, Amaro T, et al. Urothelial bladder cancer progression: lessons learned from the bench. J Cancer Metastasis Treat. 2015. https://doi.org/10.4103/2394-4722.157377.
Narayanan S, Srinivas S. Incorporating VEGF-targeted therapy in advanced urothelial cancer. Ther Adv Med Oncol. 2017. https://doi.org/10.1177/1758834016667179.
Fus ŁP, Górnicka B. Role of angiogenesis in urothelial bladder carcinoma. Cent European J Urol. 2016. https://doi.org/10.5173/ceju.2016.830.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991. https://doi.org/10.1056/NEJM199101033240101.
Bochner BH, Cote RJ, Weidner N, Groshen S, Chen S, Skinner DG, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. JNCI: Journal of the National Cancer Institute. 1995; https://doi.org/10.1093/jnci/87.21.1603 .
El Gehani K, Al-Kikhia L, Mansuri N, Syrjänen K, Al-Fituri O, Adam E. Angiogenesis in urinary bladder carcinoma as defined by microvessel density (MVD) after immunohistochemical staining for Factor VIII and CD31. Libyan J Med. 2011. https://doi.org/10.3402/ljm.v6i0.6016.
Huang J, Ma X, Chen X, Liu X, Zhang B, Minmin L, et al. Microvessel density as a prognostic factor in bladder cancer: a systematic review of literature and meta-analysis. Cancer Biomark. 2014. https://doi.org/10.3233/CBM-140417.
Friedrich MG, Toma MI, Petri S, Cheng JC, Hammerer P, Erbersdobler A, et al. Expression of Maspin in non-muscle invasive bladder carcinoma: correlation with tumor angiogenesis and prognosis. Eur Urol. 2004. https://doi.org/10.1016/j.eururo.2003.12.005.
Lin Z, Chen S, Ye C, Zhu S. Nitric oxide synthase expression in human bladder cancer and its relation to angiogenesis. Urol Res. 2003. https://doi.org/10.1007/s00240-003-0302-9.
Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med. 2007. https://doi.org/10.1007/s00109-007-0221-2.
•• Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018; https://doi.org/10.1007/s10456-018-9613-x. A comprehensive guideline providing a global review of in vivo, ex vivo, and in vitro angiogenic assays that are available for the evaluation of angiogenesis and highlights critical aspects that are relevant for their execution and proper interpretation.
Sardari Nia P, Colpaert C, Vermeulen P, Weyler J, Pezzella F, Van Schil P, et al. Different growth patterns of non-small cell lung cancer represent distinct biologic subtypes. Ann Thorac Surg. 2008. https://doi.org/10.1016/j.athoracsur.2007.08.054.
Fonsatti E, Nicolay HJM, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res. 2010. https://doi.org/10.1093/cvr/cvp332.
Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, et al. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. 1995;1(12):1623–34.
Fonsatti E, Jekunen AP, Kairemo KJ, Coral S, Snellman M, Nicotra MR, et al. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin Cancer Res. 2000;6(5):2037–43.
Miller DW, Graulich W, Karges B, Stahl S, Ernst M, Ramaswamy A, et al. Elevated expression of endoglin, a component of the TGF-β-receptor complex, correlates with proliferation of tumor endothelial cells. Int J Cancer. 1999. https://doi.org/10.1002/(sici)1097-0215(19990517)81:4%3C568::aid-ijc11%3E3.0.co;2-x.
Fonsatti E, Vecchio LD, Altomonte M, Sigalotti L, Nicotra MR, Coral S, et al. Endoglin: an accessory component of the TGF-β-binding receptor-complex with diagnostic, prognostic, and bioimmunotherapeutic potential in human malignancies. J Cell Physiol. 2001. https://doi.org/10.1002/jcp.1095.
Wikström P, Lissbrant IF, Stattin P, Egevad L, Bergh A. Endoglin (CD105) is expressed on immature blood vessels and is a marker for survival in prostate cancer. Prostate. 2002. https://doi.org/10.1002/pros.10083.
Miyata Y, Sagara Y, Watanabe S, Asai A, Matsuo T, Ohba K, et al. CD105 is a more appropriate marker for evaluating angiogenesis in urothelial cancer of the upper urinary tract than CD31 or CD34. Virchows Arch. 2013. https://doi.org/10.1007/s00428-013-1463-8.
Agrawal U, Mishra AK, Salgia P, Verma S, Mohanty NK, Saxena S. Role of tumor suppressor and angiogenesis markers in prediction of recurrence of non muscle invasive bladder cancer. Pathol Oncol Res. 2011. https://doi.org/10.1007/s12253-010-9287-1.
Mousa HM, Jassem AN and Hussain MJ. The Endoglin (CD105) Expression as a marker of tumour vasculature in urinary bladder tumours of Iraqi patients. Mesopotamia Environ J. 2017;SIC:93–8.
• Ollauri-Ibáñez C, Ayuso-Íñigo B and Pericacho M. Hot and cold tumors: is endoglin (CD105) a potential target for vessel normalization?. Cancers. 2021; https://doi.org/10.3390/cancers13071552 . This article highlights the role of endoglin as a predictor of immunosuppressive microenvironment and its potential usefulness in predicting patient response to immunotherapy.
•• Adamo A, Bruno A, Menallo G, Francipane MG, Fazzari M, Pirrone R, et al. Blood vessel detection algorithm for tissue engineering and quantitative histology. Ann Biomed Eng. 2022; https://doi.org/10.1007/s10439-022-02923-2. A blood vessel detection algorithm is provided as an open-source application working on different operating systems. This application facilitates histopathological quantification of blood vessels in immunohistochemistry sections.
Zee Y, O’Connor JPB, Parker GJM, Jackson A, Clamp AR, Taylor MB, et al. Imaging angiogenesis of genitourinary tumors. Nat Rev Urol. 2010. https://doi.org/10.1038/nrurol.2009.262.
Agrawal S. The basic molecular biology of angiogenesis and its implication in anticancer therapeutics. Arch Int Surg. 2015. https://doi.org/10.4103/2278-9596.167472.
Bernardini S, Fauconnet S, Chabannes E, Henry PC, Adessi G, Bittard H. Serum levels of vascular endothelial growth factor as a prognostic factor in bladder cancer. J Urol. 2001. https://doi.org/10.1016/S0022-5347(05)65752-7.
Pignot G, Bieche I, Vacher S, Güet C, Vieillefond A, Debré B, et al. Large-scale Real-time reverse transcription-PCR approach of angiogenic pathways in human transitional cell carcinoma of the bladder: identification of VEGFA as a major independent prognostic marker. Eur Urol. 2009. https://doi.org/10.1016/j.eururo.2008.05.027.
Huang Z, Zhang M, Chen G, Wang W, Zhang P, Yue Y, et al. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int J Oncol. 2019. https://doi.org/10.3892/ijo.2019.4729.
Sankhwar M, Sankhwar SN, Abhishek A, Rajender S. Clinical significance of the VEGF level in urinary bladder carcinoma. Cancer biomark. 2015. https://doi.org/10.3233/CBM-150478.
Mori K, Schuettfort VM, Katayama S, Laukhtina E, Pradere B, Quhal F, et al. The value of preoperative plasma VEGF levels in urothelial carcinoma of the bladder treated with radical cystectomy. Eur Urol focus. 2022. https://doi.org/10.1016/j.euf.2021.08.006.
• Ghafouri S, Burkenroad A, Pantuck M, Almomani B, Stefanoudakis D, Shen J, et al. VEGF inhibition in urothelial cancer: the past, present and future. World J Urol. 2021; https://doi.org/10.1007/s00345-020-03213-z. A review article summarising the current therapeutic challenges associated with anti-angiogenic agents in BCs.
Suzuki K, Morita T, Tokue A. Vascular endothelial growth factor-C (VEGF-C) expression predicts lymph node metastasis of transitional cell carcinoma of the bladder. Int J Urol. 2005. https://doi.org/10.1111/j.1442-2042.2005.01010.x.
Nadaoka J, Horikawa Y, Saito M, Kumazawa T, Inoue T, Narita S, et al. Prognostic significance of HIF-1α polymorphisms in transitional cell carcinoma of the bladder. Int J Cancer. 2008. https://doi.org/10.1002/ijc.23256.
Szarvas T, Jäger T, Tötsch M, Vom Dorp F, Kempkensteffen C, Kovalszky I, et al. Angiogenic switch of Angiopietins-Tie2 System and its prognostic value in bladder cancer. Clin Cancer Res. 2008. https://doi.org/10.1158/1078-0432.CCR-08-0677.
Zaravinos A, Volanis D, Lambrou GI, Delakas D, Spandidos DA. Role of the angiogenic components, VEGFA, FGF2, OPN and RHOC, in urothelial cell carcinoma of the urinary bladder. Oncol Rep. 2012. https://doi.org/10.3892/or.2012.1948.
Huang Y, Qi W, He A, Sun Y, Shen Z, Yao Y. Prognostic value of tissue vascular endothelial growth factor expression in bladder cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013. https://doi.org/10.7314/APJCP.2013.14.2.645.
Theodoropoulos VE, Lazaris AC, Sofras F, Gerzelis I, Tsoukala V, Ghikonti I, et al. Hypoxia-inducible factor 1α expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004. https://doi.org/10.1016/j.eururo.2004.04.008.
Donmez G, Sullu Y, Baris S, Yildiz L, Aydin O, Karagoz F, et al. Vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9), and thrombospondin-1 (TSP-1) expression in urothelial carcinomas. Pathol Res Pract. 2009. https://doi.org/10.1016/j.prp.2009.07.015.
Chen W, Hung W, Kang W, Huang Y, Su Y, Yang C, et al. Overexpression of cyclooxygenase-2 in urothelial carcinoma in conjunction with tumor-associated-macrophage infiltration, hypoxia-inducible factor-1α expression, and tumor angiogenesis. APMIS. 2009. https://doi.org/10.1111/j.1600-0463.2008.00004.x.
Miao C, Liang C, Zhu J, Xu A, Zhao K, Hua Y, et al. Prognostic role of matrix metalloproteinases in bladder carcinoma: a systematic review and meta-analysis. Oncotarget. 2017; https://doi.org/10.18632/oncotarget.15907.
Tuncbilek N, Kaplan M, Altaner S, Atakan IH, Sut N, Inci O, et al. Value of dynamic contrast-enhanced MRI and correlation with tumor angiogenesis in bladder cancer. AJR Am J Roentgenol. 2009. https://doi.org/10.2214/AJR.08.1332.
Hassanien OA, Abouelkheir RT, Abou El-Ghar MI, Badawy ME, El Gamal SA, El-Hamid MA. Dynamic contrast-enhanced magnetic resonance imaging as a diagnostic tool in the assessment of tumour angiogenesis in urinary bladder cancer. Can Assoc Radiol J. 2019. https://doi.org/10.1016/j.carj.2018.11.004.
Barentsz JO, Berger-Hartog O, Witjes JA, Hulsbergen-van der Kaa C, Oosterhof GO, VanderLaak JA, et al. Evaluation of chemotherapy in advanced urinary bladder cancer with fast dynamic contrast-enhanced MR imaging. Radiology. 1998. https://doi.org/10.1148/radiology.207.3.9609906.
Dobson MJ, Carrington BM, Collins CD, Ryder WDJ, Read G, Hutchinson CE, et al. The assessment of irradiated bladder carcinoma using dynamic contrast-enhanced MR imaging. Clin Radiol. 2001. https://doi.org/10.1053/crad.2000.0560.
• Fu Z, Zhang J, Lu Y, Wang S, Mo X, He Y, et al. Clinical applications of superb microvascular imaging in the superficial tissues and organs: a systematic review. Acad Radiol. 2021; https://doi.org/10.1016/j.acra.2020.03.032. The article depicts the role of the innovative SMI in assessing microvessels and their distribution in detail. The technique is becoming acceptable and is predicted to provide valuable diagnostic and prognostic information in diseases associated with angiogenesis.
Artul S, Nseir W, Armaly Z, Soudack M. Superb microvascular imaging: added value and novel applications. J Clin Imaging Sci. 2017. https://doi.org/10.4103/jcis.JCIS_79_17.
Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, et al. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res. 2004. https://doi.org/10.1158/0008-5472.CAN-04-0505.
Patel NS, Dobbie MS, Rochester M, Steers G, Poulsom R, Le Monnier K, et al. Up-regulation of endothelial delta-like 4 expression correlates with vessel maturation in bladder cancer. Clin Cancer Res. 2006. https://doi.org/10.1158/1078-0432.CCR-06-0285.
Golshani R, Lopez L, Estrella V, Kramer M, Iida N, Lokeshwar VB. Hyaluronic acid synthase-1 expression regulates bladder cancer growth, invasion, and angiogenesis through CD44. Cancer Res. 2008. https://doi.org/10.1158/0008-5472.CAN-07-2140.
Miyake M, Fujimoto K, Tanaka N, Hirao Y, Anai S, Ohnishi S, et al. Heme oxygenase-1 promotes angiogenesis in urothelial carcinoma of the urinary bladder. Oncol Rep. 2011. https://doi.org/10.3892/or.2010.1125.
Shirotake S, Miyajima A, Kosaka T, Tanaka N, Maeda T, Kikuchi E, et al. Angiotensin II type 1 receptor expression and microvessel density in human bladder cancer. Urology. 2011. https://doi.org/10.1016/j.urology.2010.11.002.
Xue Y, Lu Q, Sun Z. CD147 overexpression is a prognostic factor and a potential therapeutic target in bladder cancer. Med Oncol. 2011. https://doi.org/10.1007/s12032-010-9582-4.
Feng C, Wu Z, Guo T, Jiang H, Guan M, Zhang Y, et al. BLCA-4 expression is related to MMP-9, VEGF, IL-1α and IL-8 in bladder cancer but not to PEDF, TNF-α or angiogenesis. Pathol Biol (Paris). 2012. https://doi.org/10.1016/j.patbio.2011.11.009.
Shimada K, Fujii T, Tsujikawa K, Anai S, Fujimoto K, Konishi N. ALKBH3 contributes to survival and angiogenesis of human urothelial carcinoma cells through NADPH Oxidase and Tweak/Fn14/VEGF signals. Clin Cancer Res. 2012. https://doi.org/10.1158/1078-0432.CCR-12-0955.
Roudnicky F, Poyet C, Otto VI, Detmar M, Wild P, Krampitz S, et al. Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A–induced angiogenesis. Cancer Res. 2013. https://doi.org/10.1158/0008-5472.CAN-12-1855.
Beckham CJ, Olsen J, Yin P, Wu C, Ting H, Hagen FK, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014. https://doi.org/10.1016/j.juro.2014.02.035.
Bertz S, Abeé C, Schwarz-Furlan S, Alfer J, Hofstädter F, Stoehr R, et al. Increased angiogenesis and FGFR protein expression indicate a favourable prognosis in bladder cancer. Virchows Arch. 2014. https://doi.org/10.1007/s00428-014-1672-9.
Feng C, Wang L, Ding G, Jiang H, Ding Q, Wu Z. BLCA1 expression is associated with angiogenesis of bladder cancer and is correlated with common pro-angiogenic factors. Int J Clin Exp Med. 2015;8(9):16259–65.
Gao Y, Wu K, Chen Y, Zhou J, Du C, Shi Q, et al. Beyond proliferation: KLF5 promotes angiogenesis of bladder cancer through directly regulating VEGFA transcription. Oncotarget. 2015; https://doi.org/10.18632/oncotarget.6101.
Tian D, Hu H, Sun Y, Tang Y, Lei M, Liu L, et al. Expression of brain-specific angiogenesis inhibitor-1 and association with p53, microvessel density and vascular endothelial growth factor in the tissue of human bladder transitional cell carcinoma. Mol Med Rep. 2015. https://doi.org/10.3892/mmr.2015.3984.
Geng J, Fan J, Wang Q, Zhang X-, Kang L, Li Q-, et al. Decreased REG1α expression suppresses growth, invasion and angiogenesis of bladder cancer. Eur J Surg Oncol. 2017; https://doi.org/10.1016/j.ejso.2017.01.013.
Roudnicky F, Dieterich LC, Poyet C, Buser L, Wild P, Tang D, et al. High expression of insulin receptor on tumour-associated blood vessels in invasive bladder cancer predicts poor overall and progression-free survival. J Pathol. 2017. https://doi.org/10.1002/path.4892.
Hou T, Zhou L, Wang L, Kazobinka G, Chen Y, Zhang X, et al. Leupaxin promotes bladder cancer proliferation, metastasis, and angiogenesis through the PI3K/AKT pathway. Cell Physiol Biochem. 2018. https://doi.org/10.1159/000491536.
Hui K, Wu S, Yue Y, Gu Y, Guan B, Wang X, et al. RASAL2 inhibits tumor angiogenesis via p-AKT/ETS1 signaling in bladder cancer. Cell Signal. 2018. https://doi.org/10.1016/j.cellsig.2018.04.006.
Guo Y, Wang J, Zhou K, Lv J, Wang L, Gao S, et al. Cytotoxic necrotizing factor 1 promotes bladder cancer angiogenesis through activating RhoC. FASEB J. 2020. https://doi.org/10.1096/fj.201903266RR.
Wang J, Guo M, Zhou X, Ding Z, Chen X, Jiao Y, et al. Angiogenesis related gene expression significantly associated with the prognostic role of an urothelial bladder carcinoma. Transl Androl Urol. 2020; https://doi.org/10.21037/tau-20-1291.
Dong Y, Ma W, Shi Z, Zhang Z, Zhou J, Li Y, et al. Role of NRP1 in bladder cancer pathogenesis and progression. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.685980.
Gao D, Niu Q, Gong Y, Guo Q, Zhang S, Wang Y, et al. Y-box binding protein 1 regulates angiogenesis in bladder cancer via miR-29b-3p-VEGFA pathway. J Oncol. 2021. https://doi.org/10.1155/2021/9913015.
Li X, Wei Z, Yu H, Xu Y, He W, Zhou X, et al. Secretory autophagy-induced bladder tumour-derived extracellular vesicle secretion promotes angiogenesis by activating the TPX2-mediated phosphorylation of the AURKA-PI3K-AKT axis. Cancer Lett. 2021. https://doi.org/10.1016/j.canlet.2021.09.036.
Li Y, Zhang K, Yang F, Jiao D, Li M, Zhao X, et al. Prognostic value of vascular-expressed PSMA and CD248 in urothelial carcinoma of the bladder. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.771036.
Ma W, Ou T, Cui X, Wu K, Li H, Li Y, et al. HSP47 contributes to angiogenesis by induction of CCL2 in bladder cancer. Cell Signal. 2021. https://doi.org/10.1016/j.cellsig.2021.110044.
Wang G, Dai Y, Li K, Cheng M, Xiong G, Wang X, et al. Deficiency of Mettl3 in bladder cancer stem cells inhibits bladder cancer progression and angiogenesis. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.627706.
Abd El-Azeem MA, Ali MA, El-Shorbagy SH. Expression of GLUT4 and FAP in urothelial bladder carcinoma: correlation with angiogenesis and clinicopathological characteristics. J Egypt Natl Canc Inst. 2022. https://doi.org/10.1186/s43046-022-00145-0.
Mori K, Schuettfort VM, Katayama S, Laukhtina E, Pradere B, Quhal F, et al. Prognostic role of preoperative vascular cell adhesion molecule-1 plasma levels in urothelial carcinoma of the bladder treated with radical cystectomy. Ann Surg Oncol. 2022. https://doi.org/10.1245/s10434-022-11575-4.
Vlachostergios PJ, Tamposis IA, Anagnostou M, Papathanassiou M, Mitrakas L, Zachos I, et al. Hypoxia-inducible factor-2-altered urothelial carcinoma: clinical and genomic features. Curr Oncol. 2022. https://doi.org/10.3390/curroncol29110681.
Xia Y, Wang X, Liu Y, Shapiro E, Lepor H, Tang M, et al. PKM2 Is essential for bladder cancer growth and maintenance. Cancer Res. 2022. https://doi.org/10.1158/0008-5472.CAN-21-0403.
Yang F, Liu X, He J, Xian S, Yang P, Mai Z, et al. Occludin facilitates tumour angiogenesis in bladder cancer by regulating IL8/STAT3 through STAT4. J Cell Mol Med. 2022. https://doi.org/10.1111/jcmm.17257.
Catto JWF, Alcaraz A, Bjartell AS, De Vere WR, Evans CP, Fussel S, et al. MicroRNA in prostate, bladder, and kidney cancer: a systematic review. Eur Urol. 2011. https://doi.org/10.1016/j.eururo.2011.01.044.
Enokida H, Yoshino H, Matsushita R, Nakagawa M. The role of microRNAs in bladder cancer. Investig Clin Urol. 2016. https://doi.org/10.4111/icu.2016.57.S1.S60.
Xie Y, Ma X, Chen L, Li H, Gu L, Gao Y, et al. MicroRNAs with prognostic significance in bladder cancer: a systematic review and meta-analysis. Sci Rep. 2017. https://doi.org/10.1038/s41598-017-05801-3.
•• Parizi PK, Yarahmadi F, Tabar HM, Hosseini Z, Sarli A, Kia N, et al. MicroRNAs and target molecules in bladder cancer. Med Oncol. 2020; https://doi.org/10.1007/s12032-020-01435-0. This is a recent comprehensive review evaluating the role of miRNAs and their possible application in the diagnosis, prognosis, and prevention of BCs.
Mitash N, Tiwari S, Agnihotri S, Mandhani A. Bladder cancer: micro RNAs as biomolecules for prognostication and surveillance. Indian J Urol. 2017. https://doi.org/10.4103/0970-1591.203412.
Su G, He Q, Wang J. Clinical values of long non-coding RNAs in bladder cancer: a systematic review. Front Physiol. 2018. https://doi.org/10.3389/fphys.2018.00652.
• Grimaldi AM, Lapucci C, Salvatore M, Incoronato M and Ferrari M. Urinary miRNAs as a diagnostic tool for bladder cancer: a systematic review. Biomedicines. 2022; https://doi.org/10.3390/biomedicines10112766 . This systematic review highlights the power of urine miRNAs as BC diagnostic markers. Some miRNA signatures are shown to have better diagnostic powers compared to others. The article also highlights the pitfalls associated with clinical application.
Shi H, Yu J, Yu J, Feng Z, Zhang C, Li G, et al. Diagnostic significance of microRNAs as novel biomarkers for bladder cancer: a meta-analysis of ten articles. World J Surg Oncol. 2017. https://doi.org/10.1186/s12957-017-1201-9.
Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013. https://doi.org/10.1038/nrurol.2013.113.
Usuba W, Urabe F, Yamamoto Y, Matsuzaki J, Sasaki H, Ichikawa M, et al. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2019. https://doi.org/10.1111/cas.13856.
• GüllüAmuran G, Tinay I, Filinte D, Ilgin C, Peker Eyüboğlu I and Akkiprik M. Urinary micro-RNA expressions and protein concentrations may differentiate bladder cancer patients from healthy controls. Int Urol Nephrol. 2020; https://doi.org/10.1007/s11255-019-02328-6. A study demonstrating the potential of a panel of exosomal miRNAs in early detection of BCs.
•• Hammouz RY, Kołat D, Kałuzińska Ż, Płuciennik E and Bednarek AK. MicroRNAs: their role in metastasis, angiogenesis, and the potential for biomarker utility in bladder carcinomas. Cancers. 2021; https://doi.org/10.3390/cancers13040891. A review article summarising the role of miRNAs in BC angiogenesis and metastasis. The role of miRNA in EMT is discussed in detail as well as the usefulness of exosomes as non-invasive tools in miRNA detection.
Xu Y, Zhou M, Wang J, Zhao Y, Li S, Zhou B, et al. Role of microRNA-27a in down-regulation of angiogenic factor AGGF1 under hypoxia associated with high-grade bladder urothelial carcinoma. Biochim Biophys Acta. 2014. https://doi.org/10.1016/j.bbadis.2014.01.007.
Yu G, Yao W, Xiao W, Li H, Xu H, Lang B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J Exp Clin Cancer Res. 2014. https://doi.org/10.1186/s13046-014-0115-4.
Wang X, Wu Q, Xu B, Wang P, Fan W, Cai Y, et al. miR-124 exerts tumor suppressive functions on the cell proliferation, motility and angiogenesis of bladder cancer by fine-tuning UHRF1. FEBS J. 2015. https://doi.org/10.1111/febs.13502.
Wu S, He H, Kang Y, Xu R, Zhang L, Zhao X, et al. MicroRNA-200c affects bladder cancer angiogenesis by regulating the Akt2/mTOR/HIF-1 axis. Transl Cancer Res. 2019; https://doi.org/10.21037/tcr.2019.10.23.
Zhou X, QI L, Tong S, Cui Y, Chen J, Huang T, et al. miR-128 downregulation promotes growth and metastasis of bladder cancer cells and involves VEGF-C upregulation. Oncol Lett. 2015; https://doi.org/10.3892/ol.2015.3689.
Wang Y, Xing Q, Liu X, Guo Z, Li C, Sun G. MiR-122 targets VEGFC in bladder cancer to inhibit tumor growth and angiogenesis. Am J Transl Res. 2016;8(7):3056–66.
Cao Z, Xu L, Zhao S, Zhu X. The functions of microRNA-124 on bladder cancer. Onco Targets and Ther. 2019. https://doi.org/10.2147/OTT.S193661.
Zhang W, Mao S, Shi D, Zhang J, Zhang Z, Guo Y, et al. MicroRNA-153 decreases tryptophan catabolism and inhibits angiogenesis in bladder cancer by targeting indoleamine 2,3-dioxygenase 1. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00619.
Wang F, Fan M, Zhou X, Yu Y, Cai Y, Wu H, et al. A positive feedback loop between TAZ and MIR-942-3p modulates proliferation, angiogenesis, epithelial-mesenchymal transition process, glycometabolism and ROS homeostasis in human bladder cancer. J Exp Clin Cancer Res. 2021. https://doi.org/10.1186/s13046-021-01846-5.
Chan T, Hsing C, Shiue Y, Huang SK, Hsieh K, Kuo Y, et al. Angiogenesis driven by the CEBPD–hsa-miR-429–VEGFA signaling axis promotes urothelial carcinoma progression. Cells. 2022. https://doi.org/10.3390/cells11040638.
Xing Y, Bai Z, Liu C, Hu S, Ruan M, Chen L. Research progress of long noncoding RNA in China. IUBMB Life. 2016. https://doi.org/10.1002/iub.1564.
Zhou Y, Chen Y, Ding W, Hua Z, Wang L, Zhu Y, et al. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR-96/FOXO3. IUBMB Life. 2018. https://doi.org/10.1002/iub.1699.
Yang H, Li G, Cheng B and Jiang R. ZFAS1 functions as an oncogenic long non-coding RNA in bladder cancer. Biosci Rep. 2018. 10.1042/BSR20180475
•• Li H, Gong X, Li Z, Qin W, He C, Xing L, et al. Role of long non-coding RNAs on bladder cancer. Front Cell Dev Biol. 2021; https://doi.org/10.3389/fcell.2021.672679. Review article highlighting the role of lncRNAs in BC pathogenesis, their correlation with clinical parameters, and their potential as effective diagnostic and prognostic parameters.
•• Mirzaei S, Paskeh MDA, Hashemi F, Zabolian A, Hashemi M, Entezari M, et al. Long non-coding RNAs as new players in bladder cancer: lessons from pre-clinical and clinical studies. Life Sci. 2022; https://doi.org/10.1016/j.lfs.2021.119948. Review article highlighting the role of lncRNAs in BC pathogenesis, their correlation with clinical parameters, and their potential as effective diagnostic and prognostic parameters.
•• Liu Q. The emerging roles of exosomal long non‐coding RNAs in bladder cancer. J Cell Mol Med. 2022; https://doi.org/10.1111/jcmm.17152. Review article highlighting the role of lncRNAs in BC pathogenesis, their correlation with clinical parameters, and their potential as effective diagnostic and prognostic parameters.
Chi H, Yang R, Zheng X, Zhang L, Jiang R and Chen J. LncRNA RP11–79H23.3 functions as a competing endogenous RNA to regulate PTEN expression through sponging hsa-miR-107 in the development of bladder cancer. Int J Mol Sci. 2018; https://doi.org/10.3390/ijms19092531.
Liu B, Gao W, Sun W, Li L, Wang C, Yang X, et al. Promoting roles of long non-coding RNA FAM83H-AS1 in bladder cancer growth, metastasis, and angiogenesis through the c-Myc-mediated ULK3 upregulation. Cell cycle. 2020; https://doi.org/10.1080/15384101.2020.1850971.
Wang Y, Zhang L, Wei N, Sun Y, Pan W and Chen Y. Silencing LINC00482 inhibits tumor-associated inflammation and angiogenesis through down-regulation of MMP-15 via FOXA1 in bladder cancer. Aging (Albany NY). 2020; https://doi.org/10.18632/aging.202247.
• Li X, Zhang C, Peng X, Li Y, Chen G, Gou X, et al. A novel risk score model based on five angiogenesis-related long non-coding RNAs for bladder urothelial carcinoma. Cancer Cell Int. 2022; https://doi.org/10.1186/s12935-022-02575-1. A recent study identifying and validating a panel of survival-related lncRNAs in BC. These findings may provide therapeutic benefits.
Xiao Y, Wang T, Cheng X, Liu F, Wu Y, Ma L, et al. LINC00958 Inhibits autophagy of bladder cancer cells via sponge adsorption of miR-625-5p to promote tumor angiogenesis and oxidative stress. Oxid Med Cell Longev. 2022. https://doi.org/10.1155/2022/2435114.
He J, Dong C, Zhang H, Jiang Y, Liu T, Man X. The oncogenic role of TFAP2A in bladder urothelial carcinoma via a novel long noncoding RNA TPRG1-AS1/DNMT3A/CRTAC1 axis. Cellular Signal. 2023. https://doi.org/10.1016/j.cellsig.2022.110527.
Kang Z, Dou Q, Huang T, Tu M, Zhong Y, Wang M, et al. An angiogenesis related lncRNA signature for the prognostic prediction of patients with bladder cancer and LINC02321 promotes bladder cancer progression via the VEGFA signaling pathway. Mol Med Rep. 2023. https://doi.org/10.3892/mmr.2022.12925.
Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang C, et al. CircHIPK3 sponges miR‐558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017; https://doi.org/10.15252/embr.201643581.
•• Yang X, Ye T, Liu H, Lv P, Duan C, Wu X, et al. Expression profiles, biological functions and clinical significance of circRNAs in bladder cancer. Mol Cancer. 2021; https://doi.org/10.1186/s12943-020-01300-8. A review article providing a comprehensive view of circRNAs and the potential clinical applications of these molecules for BC diagnosis, prognosis, and targeted therapy.
Zhong Z, Huang M, Lv M, He Y, Duan C, Zhang L, et al. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017. https://doi.org/10.1016/j.canlet.2017.06.027.
Cao W, Zhao Y, Wang L, Huang X. Circ0001429 regulates progression of bladder cancer through binding miR-205-5p and promoting VEGFA expression. Cancer biomark. 2019. https://doi.org/10.3233/CBM-182380.
Mao W, Huang X, Wang L, Zhang Z, Liu M, Li Y, et al. Circular RNA hsa_circ_0068871 regulates FGFR3 expression and activates STAT3 by targeting miR-181a-5p to promote bladder cancer progression. J Exp Clin Cancer Res. 2019. https://doi.org/10.1186/s13046-019-1136-9.
Wei Y, Zhang Y, Meng Q, Cui L, Xu C. Hypoxia-induced circular RNA has_circRNA_403658 promotes bladder cancer cell growth through activation of LDHA. Am J Transl Res. 2019;11(11):6838–49.
Wang L, Li H, Qiao Q, Ge Y, Ma L and Wang Q. Circular RNA circSEMA5A promotes bladder cancer progression by upregulating ENO1 and SEMA5A expression. Aging. 2020; https://doi.org/10.18632/aging.103971.
• Li X, Peng X, Zhang C, Bai X, Li Y, Chen G, et al. bladder cancer‐derived small extracellular vesicles promote tumor angiogenesis by inducing HBP‐related metabolic reprogramming and SerRS O‐GlcNAcylation in endothelial cells. Adv Sci. 2022; https://doi.org/10.1002/advs.202202993. A study exploring the role of small extracellular vesicles in a nutrient-deprived TME and its effect on metabolic symbiosis in BC. The potential of metabolic reprogramming to target angiogenesis in BC is explored.
Liu X, Li H, Che N, Zheng Y, Fan W, Li M, et al. HBXIP accelerates glycolysis and promotes cancer angiogenesis via AKT/mTOR pathway in bladder cancer. Exp Mol Pathol. 2021. https://doi.org/10.1016/j.yexmp.2021.104665.
Lai C, Wang H, Chang H, Tsai Y, Tsai W, Lee C, et al. Enhancement of farnesoid X receptor inhibits migration, adhesion and angiogenesis through proteasome degradation and VEGF reduction in bladder cancers. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23095259.
Chien-Rui Lai, Yu-Ling T, Tsai W, Chen T, Hsin-Han C, Chih-Ying Changchien, et al. Farnesoid X receptor overexpression decreases the migration, invasion and angiogenesis of human bladder cancers via AMPK activation and cholesterol biosynthesis inhibition. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14184398.
Gakis G. The role of inflammation in bladder cancer. In: Aggarwal B, Sung B and Gupta S (eds) Inflammation and Cancer. Basel: Springer Basel, 2014, p.183–96. https://doi.org/10.1007/978-3-0348-0837-8_8
Zhu Z, Shen Z, Xu C. Inflammatory pathways as promising targets to increase chemotherapy response in bladder cancer. Mediators Inflamm. 2012. https://doi.org/10.1155/2012/528690.
Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017. https://doi.org/10.1186/s13045-017-0430-2.
Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol. 2000. https://doi.org/10.1046/j.1442-2042.2000.00190.x.
Takeuchi H, Tanaka M, Tanaka A, Tsunemi A, Yamamoto H. Predominance of M2-polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncol Lett. 2016. https://doi.org/10.3892/ol.2016.4392.
Wu H, Zhang X, Han D, Cao J, Tian J. Tumour-associated macrophages mediate the invasion and metastasis of bladder cancer cells through CXCL8. PeerJ. 2020. https://doi.org/10.7717/peerj.8721.
Sui X, Lei L, Chen L, Xie T and Li X. Inflammatory microenvironment in the initiation and progression of bladder cancer. Oncotarget. 2017; https://doi.org/10.18632/oncotarget.21565.
Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers. 2016. https://doi.org/10.1155/2016/8345286.
Karashima T, Sweeney P, Kamat A, Huang S, Kim SJ, Bar-Eli M, et al. Nuclear factor-kappaB mediates angiogenesis and metastasis of human bladder cancer through the regulation of interleukin-8. Clin Cancer Res. 2003;9(7):2786–97.
Chen M, Lin P, Wu C, Chen W, Wu C. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0061901.
Feng C, Wang P, Ding Q, Guan M, Zhang Y, Jiang H, et al. Expression of pigment epithelium-derived factor and tumor necrosis factor-α is correlated in bladder tumor and is related to tumor angiogenesis. Urol Oncol. 2013. https://doi.org/10.1016/j.urolonc.2010.12.001.
Inoue K, Slaton JW, Kim SJ, Perrotte P, Eve BY, Bar-Eli M, et al. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000;60(8):2290–9.
Gunes M, Gecit I, Pirincci N, Kemik AS, Purisa S, Ceylan K, et al. Plasma human neutrophil proteins-1, -2, and -3 levels in patients with bladder cancer. J Cancer Res Clin Oncol. 2013. https://doi.org/10.1007/s00432-012-1305-0.
Gai J, Wahafu W, Song L, Ping H, Wang M, Yang F, et al. Expression of CD74 in bladder cancer and its suppression in association with cancer proliferation, invasion and angiogenesis in HT-1376 cells. Oncol Lett. 2018. https://doi.org/10.3892/ol.2018.8309.
Peng Y, Li L, Huang M, Duan C, Zhang L, Chen J. Angiogenin interacts with ribonuclease inhibitor regulating PI3K/AKT/mTOR signaling pathway in bladder cancer cells. Cell Signal. 2014. https://doi.org/10.1016/j.cellsig.2014.08.021.
Szymańska B, Sawicka E, Matuszewski M, Dembowski J, Piwowar A. The dependence between urinary levels of angiogenesis factors, 8-Iso-prostaglandin F2α, ɣ-synuclein, and interleukin-13 in patients with bladder cancer: a pilot study. J Oncol. 2020. https://doi.org/10.1155/2020/4848752.
Szymanska B, Sawicka E, Jurkowska K, Matuszewski M, Dembowski J and Piwowar A. The relationship between interleukin-13 and angiogenin in patients with bladder cancer. J Physiol Pharmacol. 2021; https://doi.org/10.26402/jpp.2021.4.13.
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999. https://doi.org/10.1016/S0002-9440(10)65173-5.
El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010. https://doi.org/10.1093/brain/awq044.
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, et al. Advanced research on vasculogenic mimicry in cancer. J Cell Mol Med. 2015. https://doi.org/10.1111/jcmm.12496.
Li B, Mao X, Wang H, Su G, Mo C, Cao K, et al. Vasculogenic mimicry in bladder cancer and its association with the aberrant expression of ZEB1. Oncol Lett. 2018. https://doi.org/10.3892/ol.2018.7975.
Schnegg CI, Yang MH, Ghosh SK, Hsu M. Induction of vasculogenic mimicry overrides VEGF-a silencing and enriches stem-like cancer cells in melanoma. Cancer Res. 2015. https://doi.org/10.1158/0008-5472.CAN-14-1855.
Wan F, Zhang S, Xie R, Gao B, Campos B, Herold-Mende C, et al. The utility and limitations of neurosphere assay, CD133 immunophenotyping and side population assay in glioma stem cell research. Brain Pathol. 2010. https://doi.org/10.1111/j.1750-3639.2010.00379.x.
Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, et al. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013. https://doi.org/10.1038/onc.2012.85.
Folberg R, Hendrix MJC, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000. https://doi.org/10.1016/S0002-9440(10)64739-6.
Yao X, Ping Y, Bian X. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell. 2011. https://doi.org/10.1007/s13238-011-1041-2.
•• Delgado-Bellido D, Serrano-Saenz S, Fernández-Cortés M and Oliver FJ. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol Cancer. 2017; https://doi.org/10.1186/s12943-017-0631-x. The opinion study provides a comprehensive and critical insight into the in vivo and in vitro assessment of VM. The study calls upon evidence from an exhaustive review of the literature and original data to argue that the majority of in vitro studies presented as VM do not correspond to this phenomenon. The study raises doubts on the validity of concluding the presence of VM in patient samples and animal models based solely on the presence of PAS+ staining. It also outlines the requirement for new biomarkers of VM and presents criteria by which VM should be defined in vitro and in vivo.
Valdivia A, Mingo G, Aldana V, Pinto MP, Ramirez M, Retamal C, et al. Fact or fiction, it is time for a verdict on vasculogenic mimicry? Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00680.
Wang S, Yu L, Ling G, Xiao S, Sun X, Song Z, et al. Vasculogenic mimicry and its clinical significance in medulloblastoma. Cancer Biol Ther. 2012. https://doi.org/10.4161/cbt.19108.
Zhou TJ, Huang XH, Gong L, Xiang L. Vasculogenic mimicry and hypoxia-inducible factor-1α expression in cervical squamous cell carcinoma. Genet Mol Res. 2016. https://doi.org/10.4238/gmr.15017396.
Sanz L, Feijoo M, Blanco B, Serrano A, Alvarez-Vallina L. Generation of non-permissive basement membranes by anti-laminin antibody fragments produced by matrix-embedded gene-modified cells. Cancer Immunol Immunother. 2003. https://doi.org/10.1007/s00262-003-0400-0.
Francescone RA, Faibish M, Shao R. A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J Vis Exp. 2011. https://doi.org/10.3791/3040.
Sood AK, Seftor EA, Fletcher MS, Gardner LMG, Heidger PM, Buller RE, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001. https://doi.org/10.1016/S0002-9440(10)64079-5.
Hess AR, Seftor EA, Gardner LMG, Carles-Kinch K, Schneider GB, Seftor REB, et al. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2). Cancer Res. 2001;61(8):3250–5.
Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci U S A. 2001. https://doi.org/10.1073/pnas.131209798.
Seftor REB, Seftor EA, Koshikawa N, Meltzer PS, Gardner LMG, Bilban M, et al. Cooperative interactions of laminin 5 γ2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61(17):6322–7.
Sharma N, Seftor REB, Seftor EA, Gruman LM, Heidger PM Jr, Cohen MB, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002. https://doi.org/10.1002/pros.10048.
Seftor REB, Seftor EA, Kirschmann DA, Hendrix MJC. Targeting the tumor microenvironment with chemically modified tetracyclines: inhibition of laminin 5 gamma2 chain promigratory fragments and vasculogenic mimicry. Mol Cancer Ther. 2002;1(12):1173–9.
Seftor EA, Meltzer PS, Kirschmann DA, Pe’er J, Maniotis AJ, Trent JM, et al. Molecular determinants of human uveal melanoma invasion and metastasis. Clin Exp Metastasis. 2002. https://doi.org/10.1023/A:1015591624171.
Hendrix MJC, Seftor REB, Seftor EA, Gruman LM, Lee LML, Nickoloff BJ, et al. Transendothelial function of human metastatic melanoma cells: role of the microenvironment in cell-fate determination. Cancer Res. 2002;62(3):665–8.
Zhou L, Chang Y, Xu L, Hoang STN, Liu Z, Fu Q, et al. Prognostic value of vascular mimicry in patients with urothelial carcinoma of the bladder after radical cystectomy. Oncotarget. 2016; https://doi.org/10.18632/oncotarget.12775.
Fujimoto A, Onodera H, Mori A, Nagayama S, Yonenaga Y, Tachibana T. Tumour plasticity and extravascular circulation in ECV304 human bladder carcinoma cells. Anticancer Res. 2006;26(1A):59–69.
Jones RA, Wang Z, Dookie S, Griffin M. The role of TG2 in ECV304-related vasculogenic mimicry. Amino Acids. 2013. https://doi.org/10.1007/s00726-011-1214-6.
Yu L, Wu S, Zhou L, Song W, Wang D. Expressions of CD133 and CD82/KAI1 in bladder urothelial carcinoma and their correlation with vasculogenic mimicry. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33(9):1336–40.
Yang Z, Chen J, Xie H, Liu T, Chen Y, Ma Z, et al. Androgen receptor suppresses prostate cancer metastasis but promotes bladder cancer metastasis via differentially altering miRNA525-5p/SLPI-mediated vasculogenic mimicry formation. Cancer Lett. 2020. https://doi.org/10.1016/j.canlet.2019.12.018.
•• Puthenveetil A and Dubey S. Metabolic reprograming of tumor-associated macrophages. Ann Transl Med. 2020; https://doi.org/10.21037/atm-20-2037. A review article examining the metabolic circuitry in TAMs, its impact on immune effector cells, and interventions aimed at rewiring the metabolic circuits to effect TAM polarisation.
• Micheel J, Safrastyan A and Wollny D. Advances in non-coding RNA sequencing. Noncoding RNA. 2021; https://doi.org/10.3390/ncrna7040070. A comprehensive review article into the history of non-coding RNAs from past to present with special emphasis on detection methods and their limitations.
• Ebrahimi N, Faghihkhorasani F, Fakhr SS, Moghaddam PR, Yazdani E, Kheradmand Z, et al. Tumor-derived exosomal non-coding RNAs as diagnostic biomarkers in cancer. Cell Mol Life Sci. 2022; https://doi.org/10.1007/s00018-022-04552-3. A review article discussing the role of extracellular vesicles as diagnostic-prognostic tools. Special emphasis on exosomes as carriers of non-coding RNAs is provided.
Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci U S A. 2017. https://doi.org/10.1073/pnas.1612906114.
Soleimani S, Shamsi M, Ghazani MA, Modarres HP, Valente KP, Saghafian M, et al. Translational models of tumor angiogenesis: a nexus of in silico and in vitro models. Biotechnol Adv. 2018. https://doi.org/10.1016/j.biotechadv.2018.01.013.
Valente KP, Khetani S, Kolahchi AR, Sanati-Nezhad A, Suleman A, Akbari M. Microfluidic technologies for anticancer drug studies. Drug Discov Today. 2017. https://doi.org/10.1016/j.drudis.2017.06.010.
Author information
Authors and Affiliations
Contributions
Ghada Elayat and Abdel Selim contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ghada Elayat, Abdel Selim, and Ivan Punev. The first draft of the manuscript was written by Ghada Elayat and Abdel Selim. Editing, reference managing, and proofreading were performed by Ghada Elayat and Ivan Punev. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Informed Consent and Human and Animal Rights
This is a review article of published literature, recommendations, and expert analyses. This article does not include new studies. Included studies have been previously published and complied with applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elayat, G., Punev, I. & Selim, A. An Overview of Angiogenesis in Bladder Cancer. Curr Oncol Rep 25, 709–728 (2023). https://doi.org/10.1007/s11912-023-01421-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01421-5