Abstract
Purpose of Review
Cardiovascular disease (CVD) is a leading cause of death and chronic disability worldwide. Yet, despite extensive intervention strategies the number of persons affected by CVD continues to rise. Thus, there is great interest in unveiling novel mechanisms that may lead to new treatments. Considering this dilemma, recent focus has turned to the neuroimmune mechanisms involved in CVD pathology leading to a deeper understanding of the brain’s involvement in disease pathology. This review provides an overview of new and salient findings regarding the neuroimmune mechanisms that contribute to CVD.
Recent Findings
The brain contains neuroimmune niches comprised of glia in the parenchyma and immune cells at the brain’s borders, and there is strong evidence that these neuroimmune niches are important in both health and disease. Mechanistic studies suggest that the activation of glia and immune cells in these niches modulates CVD progression in hypertension and heart failure and contributes to the inevitable end-organ damage to the brain.
Summary
This review provides evidence supporting the role of neuroimmune niches in CVD progression. However, additional research is needed to understand the effects of prolonged neuroimmune activation on brain function.
Similar content being viewed by others
Introduction
The brain was once thought to be immune privileged, but it is now recognized to contain major immune niches along its borders that result from the infiltration of immune cells into the choroid plexus, meninges, and spinal cord. This occurs in the absence of inflammation, suggesting that in addition to a role in disease pathology [1], these immune niches are important for brain development [2] and homeostasis [3]. Over the last two decades, researchers have demonstrated long-range communication between the brain and immune system [4] via drainage of cerebrospinal fluid (CSF) and immune cells drain through meningeal lymphatic vessels into the deep cervical lymph nodes. More recent evidence has shown that the skull bone marrow serves an important role in central nervous system (CNS) immune surveillance in normal physiology and the response to injury [5]. Furthermore, a number of cytokines and pro-inflammatory signaling pathways are implicated in hypertension and other cardiovascular diseases (CVD) [6]. Thus, rather than being shielded from the immune system, the brain maintains a dynamic and functional relationship with the immune system for the homeostatic regulation of the brain and the body.
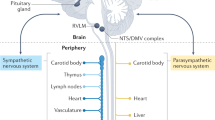
Although general neuroinflammation is important in CVD pathology [7], we lack a comprehensive understanding of the cell-specific changes to neuroimmune niches during CVD. Here, we will review the major functions of these neuroimmune niches and their role in CVD (Fig. 1). However, we will not include a discussion on neural control of the peripheral immune system, since it has been extensively reviewed elsewhere [8,9,10].
Neuroimmune niches respond to and contribute to the effects of cardiovascular disease (CVD) in the brain. Meninges: Aging is associated with B-cell expansion in the dura as well as changes to T-cell localization in the dura caused by decreasing expression of the homing receptor CCR7 and by increasing the density of FOXP3+ T-cells that reduce immune responses like waste clearing. Increased expression of VCAM1 in aging lymphatic vessels leads to impaired lymphatic drainage, which may also contribute to T cell accumulation in the aging dura. In salt-sensitive hypertension (HTN), γδT17 cells in the dura release the pro-inflammatory cytokine IL-17 which enters the cerebrospinal fluid through a disrupted arachnoid barrier. Skull bone marrow: After stroke, skull bone marrow-derived neutrophils migrate through direct vascular channels into the dura and later to the infarct site. Border associated macrophages: HTN, stroke, and obesity can contribute to blood–brain barrier breakdown by increasing expression of VEGF or pro-inflammatory cytokines such as TNFα and IL-1β. Accumulation of toxic protein aggregates like amyloid B can increase ROS causing impaired neurovascular function. Astrocytes and microglia: Astrocyte exposure to a high fat high sugar diet can thicken the basement membrane around cerebral blood vessels altering blood flow. Ang II can cross the blood–brain barrier and contribute to reduced glutamate reuptake altering sympathetic output. Salt loading in rodents increases astrocyte release of ATP contributing to high blood pressure. Microglia can increase sympathetic output indirectly by release of pro-inflammatory cytokines or directly by decreasing expression of PDGFB thereby altering K+ channel biology in neurons
Immune Surveillance of the Central Nervous System
Although microglia are the most abundant brain-resident immune cell, both microglia and astrocytes serve as the main immunocompetent cells within the CNS parenchyma. Both cell types are capable of cytokine release [11, 12], phagocytosing cell debris and toxic aggregates [13,14,15], and expression of major histocompatibility complex class II (MHCII) for antigen presentation [16, 17]. Indeed, these glial cells leverage their extensive arborizing processes to surveil their immediate neuronal and vascular environments. Specifically, microglia surveil the brain by extending and retracting their processes [18] while astrocytes form a syncytium with neighboring astrocytes to monitor neurotransmission [19] and vascular tone [20]. Reciprocal communication between microglia and astrocytes is necessary for the clearance of toxic protein aggregates. For example, microglia and astrocyte co-cultures clear amyloid-beta (Aβ) and α-synuclein more effectively than either monoculture alone [21]. Tunneling nanotubes mediate a direct and physical communication between microglia and astrocytes to facilitate microglial clearance of protein aggregates from astrocytes [21]. This reciprocal communication for the clearance of brain waste products is necessary, as the accumulation of toxic protein aggregates in the brain is linked to CVD and dementia [22].
Adjacent to the parenchyma, the brain is endowed with a specialized subset of myeloid cells residing in the choroid plexus, leptomeninges, and perivascular spaces. Collectively, these three populations of macrophages are known as brain border associated macrophages (BAMs). Although BAMs arise from the same yolk-sac-derived erythro-myeloid progenitor cells as microglia, the two cell types diverge by embryonic day 12.5 [23].
BAMs in the leptomeninges and perivascular spaces establish a stable and self-renewing population with minimal exchange from blood-derived cells, whereas macrophages in the choroid plexus undergo continuous exchange with peripheral hematopoietic stem cells (HSC) [24]. A recent study found that in the absence of disease, BAMs play major roles in regulating arterial vasomotion, extracellular matrix remodeling, and CSF flow [25••]. While much remains to be explored regarding the homeostatic actions of BAMs, evidence supports a role for BAMs in CVD including cerebral amyloid angiopathy [26•] and hypertension-induced neurovascular dysfunction [27]. Moreover, BAMs were recently identified as key contributors to Aβ-immunotherapy-induced microhemorrhages [28•].
The dura is the outermost and thickest layer of the meninges and serves as a major site of lymphatic drainage for the CSF [29]. Dural immune cells, particularly sinus-associated antigen-presenting cells, are uniquely positioned to detect brain-derived antigens found in the CSF flowing through the dural sinuses [30••]. Functional dural lymphatic vessels [31] drain these brain-derived antigens and immune cells to the deep cervical lymph nodes [32]. The dural neuroimmune niche comprises innate and adaptive immune cells that are vastly heterogenous both in their cellular make up and spatial distribution. Though largely predominated by macrophages, a wide variety of immune cells can be found throughout the dura, including neutrophils, B-cells, T-cells, natural killer cells, and dendritic cells [30••]. These immune cells are spatially localized to different sub-compartments depending on their function. For example, dural macrophages are closely associated with dural blood vessels [33]; T cells are predominantly found surrounding the dural sinuses [30••]; and pro-, pre-, immature, and mature B cells are found adjacent to and within dural blood and lymphatic vessels [34•, 35]. Fenestration of dural vessels allows for a large portion of dural immune cells to be trafficked from the periphery [36], including IgA-producing plasma cells educated in the gut and trafficked to the meninges to prevent CNS infection from blood pathogens [37].
Studies over the past 5 years have demonstrated the existence of direct channels between the dura and skull bone marrow [38••, 39••, 40••]. The discovery of dura-to-skull bone marrow channels suggests that brain-derived antigens in the CSF can influence HSC expansion and immune cell function in the skull bone marrow [40••, 41•]. Particularly, the skull bone marrow has been found to supply the dura with myeloid cells, including monocytes, neutrophils, dendritic cells, and macrophages [38••] as well as B cells and B cell progenitors [35]. These recent findings are paving the way for future discoveries, and much remains to be determined. For example, the cues that regulate the functional communication between the dura and skull bone marrow, and particularly the effect of disease on this communication, require further investigation.
Brain Immune Niches and CVD
Brain Parenchyma: Astrocytes and Microglia
A growing body of literature supports that astrocytes contribute to blood pressure elevation, a major risk factor for developing several types of CVD [42]. To this point, astrocytes in the hypothalamus can detect changes in diet and in turn cause major alterations to their neighboring vasculature. For example, in response to a high-fat/high-sugar diet, astrocytes secrete vascular endothelial growth factor (VEGF), causing an increase in hypothalamic angiogenesis and a thickening of the endothelial cell basement membrane prior to the development of hypertension [43]. In the paraventricular nucleus of the hypothalamus (PVN), reactive astrocyte-specific release of adenosine triphosphate (ATP) contributes to osmotic sensing and signaling after salt loading [44], which may increase blood pressure by promoting purinergic sympathoexcitation [45]. PVN astrocytes also respond to angiotensin II (Ang II), a hormone involved in human hypertension, by decreasing glutamate reuptake subsequently increasing the basal firing rate of PVN neurons and sympathetic output [46]. Inhibition of the Ang II receptor type 1 (AT1R) mitigates the effects on astrocyte reactivity [47].
Astrocytes are also crucial to the proposed mechanisms of brain waste clearance. In the glymphatic system model, glia provide the unidirectional waste clearance via convective fluid transport that is mediated by aquaporin-4, an astrocyte-specific water channel [48,49,50]. Alternatively, in the intramural peri-arterial drainage (IPAD) model, spontaneous smooth muscle contractions drive the clearance of soluble waste along the basement membrane of capillaries and arteries [51]. Computational modeling demonstrated that increased astrocyte coverage of an arteriole could also increase IPAD [52]. Despite the debate as to the relative importance and contribution of these two mechanisms, both are relevant to CVD, as astrocyte dysfunction leads to accumulation of waste products promoting neurotoxicity and neurodegeneration [53]. For example, impaired CSF flow and reduced solute in young spontaneously hypertensive rats (SHR) coincides with a decrease in parenchymal astrocyte area in the cortex [54].
Microglia also participate in the pathogenesis of hypertension. PVN microglial activation and pro-inflammatory cytokine release are associated with blood pressure elevation in various models of hypertension [55, 56], as well as myocardial infarction [57]. The pathology of both conditions is attenuated by treatment with minocycline, a non-specific inhibitor of microglial activation [55, 58], or by complete depletion of microglia [59]. Factors regulating neuroinflammation were also found to attenuate the cardiovascular pathology in both heart failure and hypertension, such as hypothalamic overexpression of the anti-inflammatory cytokine IL-10 [55], infusion of TGF-β into the cerebral ventricles [60], or the specific blockade of pro-inflammatory signaling [61]. More recently, the communication between microglia and neurons was found to be a key mediator of blood pressure elevation [62••]. In this study, microglia-derived PDGF-B acts via neuronal PDGFRα to regulate the basal sympathetic tone of PVN pre-sympathetic neurons [62••].
Single-cell RNA sequencing from multiple independent researchers has consistently shown that microglia do not express AT1R [63,64,65,66,67,68]. Paradoxically, although microglia lack AT1R, they become activated in response to Ang II stimulation [59]. This may be an indirect pro-inflammatory effect of Ang II on nearby neurons. Alternatively, microglia may begin to express the AT1R following a primary insult [69, 70•], which interacts with the toll-like receptor 4 (TLR4) receptor to promote neuroinflammation [71, 72]. However, other members of the renin-angiotensin system (RAS) may also drive microglial responses; for example, prorenin stimulation induces a pro-inflammatory response [56], whereas stimulation with angiotensin-(1–7) can elicit anti-inflammatory effects [73].
In short, astrocytes are capable brain resident sensors and actuators in response to peripheral signals that contribute to hypertension and metabolic disease. Microglial activation states and microglia-derived cytokines are clearly important for CVD progression. However, much remains to be determined regarding the astrocyte-to-microglia bidirectional communication in CVD.
Parenchymal Borders: Border Associated Macrophages
BAMs reside at the brain borders and serve to protect and support the interface between brain and periphery [74]. BAMs are continuously exposed to CSF due to their localization and likely monitor and regulate the CSF milieu. Indeed, BAMs rapidly phagocytose molecules such as dextran and ovalbumin delivered into the brain or the cerebral ventricles [75]. They also control CSF flow by regulating arterial vasomotion [25••], which is relevant for conditions like cerebral amyloid angiopathy, a progressive accumulation of amyloid in the leptomeninges and superficial cerebral vessels [76]. The hypothesis that amyloid accumulation in cerebral blood vessels is the result of reduced brain clearance [77] is supported by the fact that BAM depletion increases vascular amyloid deposits in mice that express a mutated form of the amyloid precursor protein [78]. Moreover, ablation of BAMs reduces CSF flow in 2-month-old 5xFAD mice and worsens amyloid accumulation, indicating that BAMs are necessary for amyloid clearance [25••]. Similarly, BAM depletion exacerbates tau pathology in the PS19 transgenic mouse model of tauopathy [79]. Yet, there seems to be a limit as to how much toxic waste can be cleared by BAMs. For instance, exposure to excess amyloid leads to toxic production of reactive oxygen species (ROS) by BAMs, thus impairing neurovascular function [80].
BAMs are also critical in mediating the amyloid-related imaging abnormalities (ARIA) that follow anti-Aβ immunotherapy [28•]. BAMs are activated by exposure to immune complexes and contributed to disruption of the blood–brain barrier (BBB) through modulation of the basement membrane [28•]. Notably, BAMs also regulate BBB disruption in hypertension [81] and stroke [82]. In various models of hypertension, BAMs and particularly AT1R activation in BAMs contribute to BBB disruption [82] as well as impairment of neurovascular and cognitive function [27, 83]. In stroke, BAM-derived VEGF contributes to BBB leakage and worsened neurological function [83].
Hypothalamic BAMs can also respond to peripheral signals. Following myocardial infarction, they modulate sympathetic activation in response to increased circulating levels of TNF and IL-1β [84]. It has also been suggested that BAMs can modulate sympathetic activation in hypertension [85]. In obesity, hypothalamic BAMs were also found to upregulate inflammatory cytokines IL-1β, IL-6, and TNFα, to exacerbate astrogliosis and promote breakdown of the BBB [86]. BAMs also play an important role in regulating the hypothalamic–pituitary–adrenal axis in systemic inflammation [87,88,89], as well as in chronic stress [90].
Recently, efforts have been placed on understanding the heterogeneity of BAMs subpopulations [91], such as those expressing Lyve1 [92•, 93••] and MHCII [25••]. This is particularly interesting, because it explores the possibility that these subpopulations function in different ways and may even serve different roles in health and disease. In addition to unraveling the distinct roles of BAM populations, it is equally important to decode the crosstalk between BAMs and neighboring cells, such as endothelial cells and glia.
Meninges and Skull Bone Marrow: The Next Frontier
A major risk factor for cardiovascular disease and several neurodegenerative diseases is aging, which affects dural immunity and meningeal lymphatic function. Specifically, aging impairs meningeal lymphatic drainage leading to the accumulation of macromolecules in the CSF that may contribute to cognitive decline [94, 95]. Changes in lymphatic endothelial cells, including increased expression of vascular cell adhesion molecule 1 (VCAM1) [30••] and altered expression of gene sets involved in immune and inflammatory responses [94, 96•, 97], are associated with the impaired meningeal lymphatic drainage in aging.
The link between the immune system and impaired meningeal lymphatic drainage was recently uncovered by a study showing that T cell accumulation in the aged meninges altered the lymphatic endothelial cells’ response to interferon-γ (IFNγ), thus impairing meningeal lymphatic function [97]. Interestingly, in aged mice, T cells not only surround the dural sinuses but are also found throughout the dura [30••], suggesting a shift in the signaling mechanisms required for homing and retention of these cells. Indeed, dural T cells in aged mice have reduced expression of CCR7, an important receptor necessary for mediating the lymphatic drainage of these cells, and deletion of Ccr7 leads to neurovascular and cognitive impairment [98•]. Furthermore, aging increases meningeal FOXP3+ Tregs, which contribute to decreased amyloid clearance and increase cognitive deficits in 5xFAD mice [98•]. Beyond T cells, aging is also associated with an accumulation of B cells [35, 99] and a reduction in B cell progenitors in the dura [100]. Thus, meningeal immunity is critical for maintaining meningeal lymphatic function and proper brain waste clearance.
Cerebrovascular events, such as ischemic stroke, increase γδT17 cells in the leptomeninges [101]. We recently found that γδT17 cells producing IL-17 in the dura mediate the neurovascular and cognitive impairment in salt-sensitive hypertensive mice [102]. Through mechanisms yet to be discovered, the arachnoid barrier underwent significant tight junction remodeling allowing IL-17 to enter the CSF [102]. Thus, as several cytokines can affect and modulate neuronal function [2], our finding that the arachnoid barrier is disrupted in hypertension has wide ranging implications, as it represents an entry path for peripheral molecules to reach the central nervous system and affect brain function in CVD.
Although the immune system in the dura and skull bone marrow are linked, they remain largely understudied in cardiovascular disease and to date, their communication has only been characterized in stroke models. Most notably, Herisson et al. demonstrated that skull bone marrow-derived neutrophils were more likely than tibia bone marrow-derived neutrophils to migrate to the site of injury after transient middle cerebral artery occlusion (tMCAO) [39••]. Considering the evidence for a skull-specific response to brain injury and functional dural immune changes due to blood pressure elevation, future research should explore the dynamic communication between the skull bone marrow and the dural immunity in the context of hypertension.
Little is known about the changes in lymphatic drainage during CVD. In stroke models, photothrombolysis but not tMCAO increases meningeal lymphangiogenesis, an effect that is modulated by VEGFR3 [103]. Lymphatic hypoplasia in Vegfr3−/− mice does not affect the outcomes following photothrombolysis, yet it exacerbates stroke severity after tMCAO [103]. On the other hand, promoting lymphatic drainage after intracerebral hemorrhage improves behavioral performance and reduces the volume of the brain hematoma [104]. Although increased lymphatic drainage after stroke may have a protective effect, with or without lymphangiogenesis, the long-term effects of increased lymphangiogenesis on immune cell populations in the dura must still be determined.
Conclusions
Here, we reviewed the growing body of literature demonstrating a functional relationship between the brain and immune system during health and in CVD. Although work detailing the interplay between the brain and immune cells is robust, there are still many knowledge gaps that remain to be addressed.
In addition to CVD, hypertension is a major risk factor for dementia and cognitive decline. In an Ang II model of hypertension, BAMs were a major source of ROS production resulting in reduced neurovascular coupling and ultimately cognitive impairment [27]. Recently, we demonstrated in a DOCA-salt model of hypertension that dural T-cell-derived IL-17 generates ROS production in BAMs via IL-17RA, which impairs neurovascular coupling and also leads to cognitive impairment [102]. Of note, in both studies, depletion of BAMs [27] or IL-17 T-cells [102] reversed cognitive impairment. These findings and others [26•, 98•] lay the groundwork for future studies to focus on the mechanisms that underlie the detrimental effects on neuronal function that result from brain border immune surveillance of peripheral to ultimately contribute to cognitive impairment and dementia.
Considering the newfound relationship between the dura and skull bone marrow, future research should investigate the hypertension-induced changes to skull bone marrow-derived immune cell populations and cytokine profiles. As mentioned above, dura to skull bone marrow communication has only been investigated in stroke models and remains poorly understood in CVD. Given the important contribution of CVD to cognitive impairment and dementia, this neuroimmune niche cannot remain unexplored. We recently found that γδT17 cells in the dura mediate the neurovascular and cognitive impairment in DOCA-salt hypertension [102], and IL-17 produced in the dura gained entry into the CSF through a disruption of the arachnoid barrier [102]. This discovery identifies a new entry path for peripheral molecules, such as Ang II, into the CNS.
CVD may also affect the relationship between microglia and astrocytes contributing to cognitive impairment. Here, we discussed the individual roles of astrocytes and microglia in blood pressure elevation. However, the two cell types are inexorably linked as they rarely function without the other and their relationship is often described as a double-edged sword. In one hypothesis, persistent microglial activation in response to neuronal damage causes the release of inflammatory cytokines that leads to neurotoxic astrocyte reactivity [104]. Further research is needed to determine how the bidirectional relationship between astrocytes and microglia exacerbates CVD and contributes to end-organ damage to the brain in chronic conditions such as hypertension. As a first step, existing single-cell RNA sequencing datasets should be leveraged to explore this communication using inference analysis such as CellChat [105].
The neuroimmune niches discussed in this review provide a new and intriguing focus for both preclinical and clinical researchers. Importantly, we must consider the function of these neuroimmune niches in regulating brain homeostatic function beyond immunity. For example, a single-nucleus RNA sequencing study revealed that choroid plexus epithelial express high levels of components from the renin angiotensin system [106••]. Considering that brain expression of renin is low [107], this recent discovery of renin-expressing cells in the choroid plexus [106••] could support the local production of Ang II in the brain. Filling the known and yet-to-be-identified knowledge gaps will be critical for mitigating the deleterious effects of CVD on a global scale.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Morimoto K, Nakajima K. Role of the immune system in the development of the central nervous system. Front Neurosci. 2019;13:916.
Salvador AF, de Lima KA, Kipnis J. Neuromodulation by the immune system: a focus on cytokines. Nat Rev Immunol. 2021;21:526–41.
Filiano AJ, Gadani SP, Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci. 2017;18:375–84.
das Neves SP, Delivanoglou N, Da Mesquita S. CNS-draining meningeal lymphatic vasculature: roles, conundrums and future challenges. Front Pharmacol 2021;12.
Mazzitelli JA, et al. Skull bone marrow channels as immune gateways to the central nervous system. Nat Neurosci. 2023;26:2052–62.
Calvillo L, Gironacci MM, Crotti L, Meroni PL, Parati G. Neuroimmune crosstalk in the pathophysiology of hypertension. Nat Rev Cardiol. 2019;16:476–90.
Carnevale D. Role of inflammatory processes in the brain-body relationship underlying hypertension. Curr Hypertens Rep. 2023;25:455–61.
Carnevale D. Neuroimmune axis of cardiovascular control: mechanisms and therapeutic implications. Nat Rev Cardiol. 2022;19:379–94.
Ahmari N, Hayward LF, Zubcevic J. The importance of bone marrow and the immune system in driving increases in blood pressure and sympathetic nerve activity in hypertension. Exp Physiol. 2020;105:1815–26.
Hu J-R, Abdullah A, Nanna MG, Soufer R. The brain–heart axis: neuroinflammatory interactions in cardiovascular disease. Curr Cardiol Rep. 2023. https://doi.org/10.1007/s11886-023-01990-8.
Choi SS, Lee HJ, Lim I, Satoh J, Kim SU. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS ONE. 2014;9: e92325.
Chhor V, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85.
Konishi H, Koizumi S, Kiyama H. Phagocytic astrocytes: emerging from the shadows of microglia. Glia. 2022;70:1009–26.
Gentleman SM. Review: microglia in protein aggregation disorders: friend or foe? Neuropathol Appl Neurobiol. 2013;39:45–50.
Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6.
Rostami J, et al. Astrocytes have the capacity to act as antigen-presenting cells in the Parkinson’s disease brain. J Neuroinflammation. 2020;17:119.
Wolf Y, et al. Microglial MHC class II is dispensable for experimental autoimmune encephalomyelitis and cuprizone-induced demyelination. Eur J Immunol. 2018;48:1308–18.
Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8.
Kiyoshi CM, Zhou M. Astrocyte syncytium: a functional reticular system in the brain. Neural Regen Res. 2019;14:595–6.
Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience. 2016;323:96–109.
Rostami J, et al. Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J Neuroinflammation. 2021;18:124.
Liu W, Wong A, Law ACK, Mok VCT. Cerebrovascular disease, amyloid plaques, and dementia. Stroke. 2015;46:1402–7.
Utz SG, et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell 2020;181:557–573.
Goldmann T, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805.
•• Drieu A, et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature. 2022;611:585–93. This study shows that BAMs can regulate the flow of CSF by promoting arterial motion. Depletion of BAMs impairs CSF perfusion and clearance.
• Uekawa K, et al. Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress. Mol Neurodegener. 2023;18:73. This study shows that BAM-derived ROS promotes neurovascular dysfunction in middle-aged Tg2576 mice. BAM depletion prevents vascular remodeling and rescues cognitive impairment.
Faraco G, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–89.
• Taylor X, et al. Amyloid-beta (Abeta) immunotherapy induced microhemorrhages are associated with activated perivascular macrophages and peripheral monocyte recruitment in Alzheimer’s disease mice. Mol Neurodegener. 2023;18:59. In this study, activated BAMs accumulate around anti-Aβ antibodies, leading to increased vascular permeability and immune cell infiltration.
Mundt S, Keller A, Greter M. The dural sinus hub: more than just a brain drain. Cell. 2021;184:858–60.
•• Rustenhoven J, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 2021;184:1000–1016. This study details the immune cell interactions in the dura in health and neurodegeneration.
Antila S, et al. Development and plasticity of meningeal lymphatic vessels. J Exp Med. 2017;214:3645–67.
Louveau A, et al. Structural and functional features of central nervous system lymphatics. Nature. 2015;523:337–41.
Sato T, Konishi H, Tamada H, Nishiwaki K, Kiyama H. Morphology, localization, and postnatal development of dural macrophages. Cell Tissue Res. 2021;384:49–58.
• Schafflick D, et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci. 2021;24:1225–34. This study found that the dura is a site for B-cell maturation and that these B-cells are not from the periphery.
Brioschi S, et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 2021;373:eabf9277.
Chen Z, Liu P, Xia X, Wang L, Li X. Living on the border of the CNS: dural immune cells in health and disease. Cell Immunol. 2022;377: 104545.
Fitzpatrick Z, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587:472–6.
•• Cugurra A, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 2021;373. This study demonstrates that the skull bone marrow replenishes the immune cells in the meninges and other brain borders, thereby challenging the current views on immune cell infiltration.
•• Herisson F, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018;21:1209–17. This was one of the first studies to demonstrate that the skull bone marrow supplies the dural immune cell populations.
•• Mazzitelli JA, et al. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat Neurosci. 2022;25:555–60. This study uses fluorescent tracers to identify ossified channels between the skull bone marrow and the dura.
• Pulous FE, et al. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat Neurosci. 2022;25:567–76. This study shows that the CSF drains into the skull bone marrow and directs hematopoiesis during infection.
Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–92.
Gruber T, et al. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021;33:1155-1170.e10.
Martins Sá RW, et al. Salt-loading promotes extracellular ATP release mediated by glial cells in the hypothalamic paraventricular nucleus of rats. Mol Cell Neurosci. 2023;124: 103806.
Ferreira-Neto HC, Ribeiro IMR, Moreira TS, Yao ST, Antunes VR. Purinergic P2 receptors in the paraventricular nucleus of the hypothalamus are involved in hyperosmotic-induced sympathoexcitation. Neuroscience. 2017;349:253–63.
Stern JE, et al. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension. 2016;68:1483–93.
Rodriguez-Ortiz CJ, et al. Angiotensin receptor blockade with olmesartan alleviates brain pathology in obese OLETF rats. Clin Exp Pharmacol Physiol. 2023;50:228–37.
Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system – a beginner’s guide. Neurochem Res. 2015;40:2583–99.
Benveniste H, et al. The glymphatic system and waste clearance with brain aging. Gerontology. 2019;65:106–19.
Hablitz LM, Nedergaard M. The glymphatic system. Curr Biol. 2021;31:R1371–5.
Kelly L, et al. Clearance of interstitial fluid (ISF) and CSF (CLIC) group-part of vascular professional interest area (PIA), updates in 2022–2023. Cerebrovascular disease and the failure of elimination of amyloid-β from the brain and retina with age and Alzheimer’s disease: opportunities for therapy. Alzheimers Dement. https://doi.org/10.1002/alz.13512.
Diem AK, Carare RO, Weller RO, Bressloff NW. A control mechanism for intra-mural peri-arterial drainage via astrocytes: how neuronal activity could improve waste clearance from the brain. PLoS ONE. 2018;13: e0205276.
Valenza M, Facchinetti R, Steardo L, Scuderi C. Altered waste disposal system in aging and Alzheimer’s disease: focus on astrocytic aquaporin-4. Front Pharmacol. 2020;10.
Mortensen KN, et al. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci. 2019;39:6365–77.
Shi P, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303.
Shi P, et al. Direct pro-inflammatory effects of prorenin on microglia. PLoS ONE. 2014;9: e92937.
Rana I, et al. Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res. 2010;1326:96–104.
Wang H-W, et al. Inhibition of inflammation by minocycline improves heart failure and depression-like behaviour in rats after myocardial infarction. PLoS ONE. 2019;14: e0217437.
Shen XZ, et al. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–16.
Li Y, et al. Brain transforming growth factor-β resists hypertension via regulating microglial activation. Stroke. 2017;48:2557–64.
Kang Y-M, et al. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol. 2011;106:473–83.
•• Bi Q, et al. Microglia-derived PDGFB promotes neuronal potassium currents to suppress basal sympathetic tonicity and limit hypertension. Immunity. 2022;55:1466-1482.e9. This study identified a novel mechanism by which microglia directly affect sympathetic output contributing to hypertension.
Van Hove H, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22:1021–35.
Vanlandewijck M, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–80.
He L, et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data. 2018;5: 180160.
Hammond TR, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253-271.e6.
Olah M, et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat Commun. 2020;11:6129.
Saunders A, et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015-1030.e16.
Sun H, et al. Angiotensin II and its receptor in activated microglia enhanced neuronal loss and cognitive impairment following pilocarpine-induced status epilepticus. Mol Cell Neurosci. 2015;65:58–67.
• Althammer F, et al. Angiotensin II–mediated neuroinflammation in the hippocampus contributes to neuronal deficits and cognitive impairment in heart failure rats. Hypertension. 2023;80:1258–73. This study indicates that microglia can express AT1aR and responds to Ang II in a model of heart failure; challenging data showing a lack of AT1aR expression in these cells.
Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE. Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. 2016;310:H404–15.
Mowry FE, Peaden SC, Stern JE, Biancardi VC. TLR4 and AT1R mediate blood-brain barrier disruption, neuroinflammation, and autonomic dysfunction in spontaneously hypertensive rats. Pharmacol Res. 2021;174: 105877.
Liu M, Shi P, Sumners C. Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. J Neurochem. 2016;136:163–71.
Kierdorf K, Masuda T, Jordão MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20:547–62.
Carare RO, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–44.
Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9.
Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. Review: Cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol. 2013;39:593–611.
Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci. 2009;106:1261–6.
Drieu A, et al. Parenchymal border macrophages regulate tau pathology and tau-mediated neurodegeneration. Life Sci Alliance. 2023;6.
Park L, et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ Res. 2017;121:258–69.
Santisteban MM, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. 2020;76:795–807.
Pedragosa J, et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol Commun. 2018;6.
Santisteban MM, Iadecola C, Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension. 2023;80:22–34.
Yu Y, et al. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–9.
Iyonaga T, Shinohara K, Mastuura T, Hirooka Y, Tsutsui H. Brain perivascular macrophages contribute to the development of hypertension in stroke-prone spontaneously hypertensive rats via sympathetic activation. Hypertens Res. 2020;43:99–110.
Lee CH, et al. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep. 2018;25:934-946.e5.
Vasilache AM, Qian H, Blomqvist A. Immune challenge by intraperitoneal administration of lipopolysaccharide directs gene expression in distinct blood–brain barrier cells toward enhanced prostaglandin E2 signaling. Brain Behav Immun. 2015;48:31–41.
Serrats J, et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106.
Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–18.
Serrats J, Grigoleit J-S, Alvarez-Salas E, Sawchenko PE. Pro-inflammatory immune-to-brain signaling is involved in neuroendocrine responses to acute emotional stress. Brain Behav Immun. 2017;62:53–63.
Mildenberger W, Stifter SA, Greter M. Diversity and function of brain-associated macrophages. Curr Opin Immunol. 2022;76: 102181.
• Karam M, Janbon H, Malkinson G, Brunet I. Heterogeneity and developmental dynamics of LYVE-1 perivascular macrophages distribution in the mouse brain. J Cereb Blood Flow Metab. 2022;42:1797–812. This study characterizes the distribution and heterogeneity of perivascular macrophages particularly focusing on the LYVE-1 subpopulation.
•• Siret C, et al. Deciphering the heterogeneity of the Lyve1+ perivascular macrophages in the mouse brain. Nat Commun. 2022;13:7366. This shows that perivascular macrophages are a heterogeneous population of cells and that those expressing Lyve1 but not express MHCII are still capable of phagocytosis.
Da Mesquita S, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–91.
Louveau A, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–91.
• Rustenhoven J, et al. Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J Exp Med. 2023;220: e20221929. This study found that aging is associated with increased meningeal IFNγ expression that impairs lymphatic function. Overexpression of IFNγ in young mice mimics the effects of aging in the meninges but is reversed by neutralizing IFNγ.
Neutzner M, Kohler C, Frank S, Killer HE, Neutzner A. Impact of aging on meningeal gene expression. Fluids and Barriers of the CNS. 2023;20:12.
• Da Mesquita S, et al. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology. Sci Adv. 2021;7:eabe4601. This study characterizes the immune cell profiles in aging mice. They found a reduction in CCR7 in T cells that leads to reduced waste clearance and cognitive impairment in aging.
Bolte AC, et al. The meningeal transcriptional response to traumatic brain injury and aging. eLife. 2023;12:e81154.
Wang Y, et al. Early developing B cells undergo negative selection by central nervous system-specific antigens in the meninges. Immunity. 2021;54:2784-2794.e6.
Benakis C, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516–23.
Santisteban MM, et al. Meningeal interleukin-17-producing T cells mediate cognitive impairment in a mouse model of salt-sensitive hypertension. Nat Neurosci. 2023. https://doi.org/10.1038/s41593-023-01497-z.
Yanev P, et al. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab. 2020;40:263–75.
Di Benedetto G, et al. Role of microglia and astrocytes in Alzheimer’s disease: from neuroinflammation to Ca2+ homeostasis dysregulation. Cells. 2022;11:2728.
Jin S, et al. Inference and analysis of cell-cell communication using Cell Chat. Nat Commun. 2021;12:1088.
•• Dani N, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184:3056-3074.e21. This study established a cellular and spatial map of the choroid plexus in embryonic, adult, and aged mice. These data will serve as an important tool for future investigation into the physiological roles of the choroid plexus.
Cruz-López EO, Uijl E, Danser AHJ. Fifty years of research on the brain renin–angiotensin system: what have we learned? Clin Sci. 2021;135:1727–31.
Acknowledgements
Current Hypertension Reports is grateful to Dr. John Hall, for his review of this manuscript.
Funding
The authors would like to acknowledge funding support from the National Institutes of Health (NIH) training grant T32 HL144446 (SMZ), grants R01 HL144941 (AK), R01 HL157584 (AK), R01 DK135764 (AK), R21 TW012635 (AK), and K22 NS123507 (MMS). Additional funding support is gratefully recognized from Burroughs Wellcome Fund Career Award at the Scientific Interface (AHJ), Chan Zuckerberg Initiative Science Diversity Leadership Grant 2022–253529 (AHJ), and American Heart Association 23SCEFIA1155595 (MMS).
Author information
Authors and Affiliations
Contributions
S.M.Z. and M.M.S. wrote the main manuscript text and S.M.Z. prepared Fig. 1. A.K. and A.H.J. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zarate, S.M., Kirabo, A., Hinton Jr., A.O. et al. Neuroimmunology of Cardiovascular Disease. Curr Hypertens Rep (2024). https://doi.org/10.1007/s11906-024-01301-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11906-024-01301-8