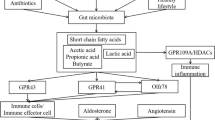
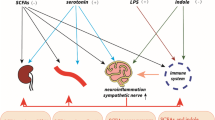
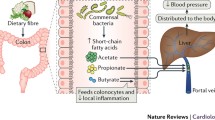
Abstract
The pathogenesis of hypertension remains elusive. Current treatments on hypertension have only achieved modest reductions. Facilitating theoretical research and looking for new therapeutic strategy are urgently needed. Besides food digestion and nutrients absorption, the gastrointestinal tract (GI) has been shown to influence the status of the central nervous system, immune system, metabolism, and cardiovascular homeostasis. Emerging findings demonstrate that endogenous factors derived from GI including gut hormones, autonomic nerve, and gut microbiota play important roles in the regulation of vascular function and/or blood pressure. Meanwhile, evidences from clinical practice and experimental study have found that intervention in GI through metabolic surgery, probiotics consumption, and dietary modification can efficiently ameliorate or even remit hypertension and related cardiometabolic diseases. Thus, we propose that GI might be an initiating organ of hypertension and a promising target for the management of hypertension. Further, illuminating this concept may aid to understand the pathogenesis and control of hypertension.

Similar content being viewed by others
References
Cowley Jr AW, Nadeau JH, Baccarelli A, Berecek K, Fornage M, Gibbons GH, et al. Report of the National Heart, Lung, and Blood Institute working group on epigenetics and hypertension. Hypertension. 2012;59(5):899–905. doi:10.1161/HYPERTENSIONAHA.111.190116.
Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15(8):737–49. doi:10.1111/dom.12085.
Sandoval DA, Seeley RJ. The microbes made me eat it. Science. 2010;328(5975):179–80. doi:10.1126/science.1188876.
• Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–66. doi:10.1038/nrn3071.
• Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60(9):1214–23. doi:10.1136/gut.2010.234708.
•• Zhu Z, Xiong S, Liu D. The gastrointestinal tract: an initial organ of metabolic hypertension? Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2016;38(5):1681–94. doi:10.1159/000443107.
Caso JR, Balanza-Martinez V, Palomo T, Garcia-Bueno B. The Microbiota and Gut-Brain Axis: Contributions to the Immunopathogenesis of Schizophrenia. Current pharmaceutical design. 2016.
Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;56:155–60. doi:10.1016/j.pnpbp.2014.08.018.
Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514(7521):237–41. doi:10.1038/nature13564.
Zhao D, Qi Y, Zheng Z, Wang Y, Zhang XY, Li HJ, et al. Dietary factors associated with hypertension. Nat Rev Cardiol. 2011;8(8):456–65. doi:10.1038/nrcardio.2011.75.
•• Afsar B, Vaziri ND, Aslan G, Tarim K, Kanbay M. Gut hormones and gut microbiota: implications for kidney function and hypertension. Journal of the American Society of Hypertension : JASH. 2016;10(12):954–61. doi:10.1016/j.jash.2016.10.007.
Yu M, Moreno C Fau-Hoagland KM, Hoagland KM, Fau-Dahly A, Dahly A, Fau-Ditter K, Ditter K, Fau-Mistry M, Mistry M, Fau-Roman RJ et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. (0263–6352 (Print)).
Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. American journal of physiology Renal physiology. 2011;301(2):F355–63. doi:10.1152/ajprenal.00729.2010.
Goud A, Zhong J, Peters M, Brook RD, Rajagopalan S. GLP-1 agonists and blood pressure: a review of the evidence. Curr Hypertens Rep. 2016;18(2):16. doi:10.1007/s11906-015-0621-6.
Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60(3):833–41. doi:10.1161/HYPERTENSIONAHA.112.195115.
Rodriguez A. Novel molecular aspects of ghrelin and leptin in the control of adipobiology and the cardiovascular system. Obesity facts. 2014;7(2):82–95. doi:10.1159/000360837.
Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159(6):1029–37. doi:10.1083/jcb.200207165.
Deng B, Fang F, Yang T, Yu Z, Zhang B, Xie X. Ghrelin inhibits AngII-induced expression of TNF-alpha, IL-8, MCP-1 in human umbilical vein endothelial cells. Int J Clin Exp Med. 2015;8(1):579–88.
Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109(18):2221–6. doi:10.1161/01.CIR.0000127956.43874.F2.
Tesauro M, Schinzari F, Rovella V, Di Daniele N, Lauro D, Mores N, et al. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension. 2009;54(5):995–1000. doi:10.1161/HYPERTENSIONAHA.109.137729.
Samuelsson AM, Clark J, Rudyk O, Shattock MJ, Bae SE, South T, et al. Experimental hyperleptinemia in neonatal rats leads to selective leptin responsiveness, hypertension, and altered myocardial function. Hypertension. 2013;62(3):627–33. doi:10.1161/HYPERTENSIONAHA.111.00691.
Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, et al. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997;10(10 Pt 1):1171–4.
Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159(6):1404–16. doi:10.1016/j.cell.2014.10.058.
Sartor DM. Sympathoinhibitory signals from the gut and obesity-related hypertension. Clinical autonomic research: official journal of the Clinical Autonomic Research Society. 2013;23(1):33–9. doi:10.1007/s10286-012-0171-9.
How JM, Wardak SA, Ameer SI, Davey RA, Sartor DM. Blunted sympathoinhibitory responses in obesity-related hypertension are due to aberrant central but not peripheral signalling mechanisms. J Physiol. 2014;592(7):1705–20. doi:10.1113/jphysiol.2013.269670.
Chen Y, Asico LD, Zheng S, Villar VA, He D, Zhou L, et al. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension. 2013;62(5):927–33. doi:10.1161/HYPERTENSIONAHA.113.01094.
Liu T, Konkalmatt PR, Yang Y, Jose PA. Gastrin decreases Na+,K+−ATPase activity via a PI 3-kinase- and PKC-dependent pathway in human renal proximal tubule cells. Am J Physiol Endocrinol Metab. 2016;310(7):E565–71. doi:10.1152/ajpendo.00360.2015.
•• Jose PA, Yang Z, Zeng C, Felder RA. The importance of the gastrorenal axis in the control of body sodium homeostasis. Exp Physiol. 2016;101(4):465–70. doi:10.1113/EP085286.
Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116(6):976–90. doi:10.1161/CIRCRESAHA.116.303604.
Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9. doi:10.1016/S0140-6736(10)62039-9.
Miroslawska A, Solbu M, Skjolsvik E, Toft I, Steigen TK. Renal sympathetic denervation: effect on ambulatory blood pressure and blood pressure variability in patients with treatment-resistant hypertension. The ReShape CV-risk study. J Hum Hypertens. 2016;30(3):153–7. doi:10.1038/jhh.2015.69.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi:10.1161/CIRCRESAHA.116.305697.
How JMY, Fam BC, Verberne AJM, Sartor DM. High-fat diet is associated with blunted splanchnic sympathoinhibitory responses to gastric leptin and cholecystokinin: implications for circulatory control. Am J Physiol-Heart C. 2011;300(3):H961–H7. doi:10.1152/ajpheart.01156.2010.
Ito S, Ohga A, Ohta T. Gastric relaxation and vasoactive intestinal peptide output in response to reflex vagal stimulation in the dog. J Physiol. 1988;404:683–93.
Petkovich BW, Vega J, Thomas S. Vagal modulation of hypertension. Curr Hypertens Rep. 2015;17(4):532. doi:10.1007/s11906-015-0532-6.
Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi:10.1136/bmj.b4567.
Li XC, Shull GE, Miguel-Qin E, Chen F, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension in NHE3-deficient mice with transgenic rescue of NHE3 in small intestines. Physiological reports. 2015;3(11):e12605. doi:10.14814/phy2.12605.
Linz D, Wirth K, Linz W, Heuer HO, Frick W, Hofmeister A, et al. Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut. Hypertension. 2012;60(6):1560–7. doi:10.1161/HYPERTENSIONAHA.112.201590.
Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6(227):227ra36. doi:10.1126/scitranslmed.3007790.
Lubcke R, Barbezat GO. Intestinal ion transport in rats with spontaneous arterial hypertension. Clin Sci. 1988;75(2):127–33.
Sanchez-Aguayo I, Torreblanca J, de La Hermosa ML, Mate A, Planas JM, Vazquez CM. Ultrastructural and functional changes in the jejunal epithelium of spontaneously hypertensive rats. Life Sci. 2001;68(18):2105–13.
Mate A, Barfull A, Hermosa AM, Gomez-Amores L, Vazquez CM, Planas JM. Regulation of sodium-glucose cotransporter SGLT1 in the intestine of hypertensive rats. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291(3):R760–7. doi:10.1152/ajpregu.00524.2005.
DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10(6):364–76. doi:10.1038/nrendo.2014.44.
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. doi:10.1073/pnas.1005963107.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. doi:10.1038/nature4441022a.
• Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. doi:10.1073/pnas.0504978102.
Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31. doi:10.1126/science.1179721.
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. doi:10.1161/Hypertensionaha.115.05315.
Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24(5):403–9. doi:10.1097/MNH.0000000000000149.
•• Kelly TN, Bazzano LA, Ajami NJ, He H, Zhao JY, Petrosino JF, et al. Gut microbiome associates with lifetime cardiovascular disease risk profile among bogalusa heart study participants. Circ Res. 2016;119(8):956–64. doi:10.1161/Circresaha.116.309219.
Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67(2):469–74. doi:10.1161/Hypertensionaha.115.06672.
Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Nitert MD, et al. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68(4):974–81. doi:10.1161/Hypertensionaha.116.07910.
•• Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64(4):897–903. doi:10.1161/Hypertensionaha.114.03469.
Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124(9):1046–58. doi:10.1161/CIRCULATIONAHA.111.030189.
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association professional education Committee of the Council for high blood pressure research. Hypertension. 2008;51(6):1403–19. doi:10.1161/HYPERTENSIONAHA.108.189141.
Ricci C, Gaeta M, Rausa E, Asti E, Bandera F, Bonavina L. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg. 2015;25(3):397–405. doi:10.1007/s11695-014-1442-4.
Lindekilde N, Gladstone BP, Lubeck M, Nielsen J, Clausen L, Vach W, et al. The impact of bariatric surgery on quality of life: a systematic review and meta-analysis. Obes Rev. 2015;16(8):639–51. doi:10.1111/obr.12294.
• Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi:10.1001/jama.292.14.1724.
Fernstrom JD, Courcoulas AP, Houck PR, Fernstrom MH. Long-term changes in blood pressure in extremely obese undergone bariatric patients who have surgery. Arch Surg-Chicago. 2006;141(3):276–83. doi:10.1001/archsurg.141.3.276.
Puzziferri N, Roshek 3rd TB, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42. doi:10.1001/jama.2014.10706.
Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003;237(6):751–6. discussion 7-8 doi:10.1097/01.SLA.0000071560.76194.11.
• Rodriguez A, Becerril S, Valenti V, Ramirez B, Martin M, Mendez-Gimenez L, et al. Sleeve gastrectomy reduces blood pressure in obese (fa/fa) Zucker rats. Obes Surg. 2012a;22(2):309–15. doi:10.1007/s11695-011-0562-3.
Ahmed AR, Rickards G, Coniglio D, Xia Y, Johnson J, Boss T, et al. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg. 2009;19(7):845–9. doi:10.1007/s11695-008-9671-z.
Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–95. doi:10.1038/ijo.2009.79.
Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2011;7(6):683–90. doi:10.1016/j.soard.2011.07.009.
Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes. 2011;35(2):153–66. doi:10.1038/ijo.2010.132.
Rodriguez A, Becerril S, Valenti V, Moncada R, Mendez-Gimenez L, Ramirez B, et al. Short-term effects of sleeve gastrectomy and caloric restriction on blood pressure in diet-induced obese rats. Obes Surg. 2012b;22(9):1481–90. doi:10.1007/s11695-012-0702-4.
Wasmund SL, Owan T, Yanowitz FG, Adams TD, Hunt SC, Hamdan MH, et al. Improved heart rate recovery after marked weight loss induced by gastric bypass surgery: two-year follow up in the Utah Obesity Study. Heart Rhythm. 2011;8(1):84–90. doi:10.1016/j.hrthm.2010.10.023.
• Zhang HX, Pu YF, Chen J, Tong WD, Cui YT, Sun F, et al. Gastrointestinal intervention ameliorates high blood pressure through antagonizing overdrive of the sympathetic nerve in hypertensive patients and rats. J Am Heart Assoc. 2014;3(5):e000929. doi:10.1161/JAHA.114.000929.
Liou AP, Paziuk M, Luevano JM, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. doi:10.1126/scitranslmed.3005687.
Lutz TA, Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. Am J Physiol-Reg I. 2014;307(11):R1275–R91. doi:10.1152/ajpregu.00185.2014.
Arora P, Reingold J, Baggish A, Guanaga DP, Wu C, Ghorbani A, et al. Weight loss, saline loading, and the natriuretic peptide system. J Am Heart Assoc. 2015;4(1):e001265. doi:10.1161/JAHA.114.001265.
Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav. 2012;107(4):476–83. doi:10.1016/j.physbeh.2012.02.013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Sources of Funding
This research was supported by the National Natural Science Foundation of China (31430042, 91639000), the National Basic Research Program of China (2013CB531205), and supported by the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sympathetic Nervous System, and Hypertension
Rights and permissions
About this article
Cite this article
Xiong, S., Li, Q., Liu, D. et al. Gastrointestinal Tract: a Promising Target for the Management of Hypertension. Curr Hypertens Rep 19, 31 (2017). https://doi.org/10.1007/s11906-017-0726-1
Published:
DOI: https://doi.org/10.1007/s11906-017-0726-1