Abstract
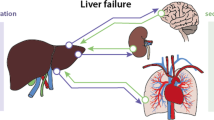
The critical care management of cirrhotic patients involves a multidisciplinary team approach, including the hepatologist and intensivist, to address life-threatening complications and to provide comprehensive care for multi-organ failure commonly seen in these patients. A systematic approach to the diagnosis and therapy of multi-organ system dysfunction is essential to optimize the intensive care management of these complex patients, with a goal to stabilize them for possible liver transplantation. This review provides a system-based approach for the intensive care management of critically ill cirrhotic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cirrhotic patients are prone to developing life-threatening complications related to dysfunction in multiple organ systems. It has been indirectly estimated that nearly 26,000 patients with cirrhosis in the USA require intensive care each year, and the mean length of hospitalization is 13.8 days [1]. In-hospital mortality has been estimated to be greater than 50 % and has shown a decline over the years with advances in critical care management. Critically ill cirrhotic patients may need hemodynamic support with vasoactive agents, mechanical ventilation, renal replacement therapy (RRT), and in some centers artificial liver replacement therapies, all of which lead to significant health care resource utilization [2]. An estimated US $3 billion, or mean charges of US $116,200 per admission, are spent on ICU care of cirrhotics [1]. Critical care management of the decompensated cirrhotic demands optimization of hepatic and extrahepatic derangements, including cardiopulmonary, neurologic, and renal dysfunction, and is essential for a successful transition out of the ICU or as a bridge to transplant [3, 4].
An organ system-based review of derangements and management strategies is outlined below.
Neurologic Derangements
Altered mental status (AMS) in cirrhotic patients usually occurs from hepatic encephalopathy (HE). However, if AMS is rapid in onset, other acute cerebral issues should be ruled out with imaging. HE is a serious complication of portal hypertension; its neuropsychiatric symptoms and neuromuscular dysfunction significantly contribute to the burden in chronic liver disease, and frequently result in hospitalization and decreased survival. HE is graded according to the West Haven criteria (grade I to grade IV) based on varying levels of consciousness, intellectual function, and behavior. Neurologic abnormalities on physical examination include asterixis, hyperactive deep tendon reflexes, and hemiplegia [4, 5].
Initial management of HE involves accurately identifying the grade of encephalopathy, with prompt transfer to the ICU and elective intubation for airway protection in HE grades III and IV. If active hematemesis is present, elective intubation should be performed with any grade of encephalopathy. Imaging of the brain should also be considered to rule out other etiologies related to the abrupt onset of altered mental status or focal neurologic deficits, including cerebrovascular accident (CVA), intracranial bleeding, or masses. Importantly, the precipitating factor of hepatic encephalopathy must be identified and treated. Common precipitating causes include gastrointestinal bleeding, infections, alkalosis or acidosis, electrolyte disturbances, overdiuresis, dehydration, placement of recent transjugular intrahepatic portosystemic shunt (TIPS), constipation, medication or dietary noncompliance, sedatives, tranquilizers, narcotics, or progressive hepatic dysfunction [5, 6]. Supportive care with IV fluid hydration, correction of electrolyte disturbances, and aspiration and fall precautions should be instituted [3, 4, 7, 8]. A lumbar puncture may be considered if there is suspicion for CNS infections, but caution has to be exercised with coagulopathy [3].
Nonabsorbable disaccharides such as lactulose or lactitol (not available in the USA) are the main pharmacologic agents to aid in the clearance of ammonia in the treatment of hepatic encephalopathy. Lactulose is administered by a nasogastric (NG) tube or as enemas in comatose patients at a dose of 40–60 g daily and titrated to two to three soft stools [3, 4]. Rifaximin is an orally administered nonabsorbable antimicrobial agent with gut selective action. A randomized double-blinded trial with rifaximin has shown to decrease overt HE recurrence and related hospitalizations, and a recent 2-year follow-up of the study showed durable results without an increase in adverse events [5, 6]. Rifaximin should be administered through an NG tube in patients with AMS at a dose of 550 mg bid. In addition to lactulose and rifaximin, other options that may be considered for refractory HE include metronidazole and zinc. Extracorporeal artificial liver support with the Molecular Adsorbent Recirculating System (MARS) has shown some effectiveness in grade 3 and 4 HE in a randomized control trial and can be considered in centers with the availability of this modality [8]. There is no role for protein restriction in the management of HE. In addition, venous ammonia levels do not correlate with the degree of encephalopathy and, therefore, should not be checked for diagnosis and therapy [4].
In some patients with AMS, large spontaneous portosystemic shunts should be considered and evaluated by imaging which can cause chronic protracted or recurrent HE. Embolization of these shunts should be considered if there is sufficient hepatic reserve [9].
Gastrointestinal System Derangements
Acute Gastrointestinal Bleeding
Bleeding from gastroesophageal varices occurs when patients have a hepatic venous pressure gradient (HVPG) of at least 10 to 12 mmHg. The risk for variceal bleeding and rebleeding correlates with severity of disease, with a lower risk of bleeding in Child–Pugh grades A and B cirrhosis, but over 30 % in Child–Pugh grade C [3, 10]. Given improved endoscopic, radiologic, and critical care interventions, hospital mortality has decreased from 42 % in 1980 to 14 % in 2000 [11].
Critical care management for acute variceal bleeding involves early and aggressive resuscitation. Airway protection with elective intubation for massive hematemesis should be performed irrespective of mental status in order to decrease the risk of aspiration. Early large-bore intravenous access to facilitate intravascular volume resuscitation is essential. At least two large-bore peripheral catheters or a central venous catheter should be placed. Blood transfusions should be initiated promptly, although a conservative rather than a liberal transfusion strategy may have been preferred. A recent study suggested better outcomes with a hemoglobin at approximately 7 g/dl, with aggressive volume resuscitation associated with increased portal pressure, rebleeding, and higher mortality [12]. In addition, cirrhotic patients are further predisposed to bleeding from thrombocytopenia and clotting factor deficiencies. Transfusion with fresh frozen plasma (FFP), cryoprecipitate, and platelets should be administered as determined by hematologic parameters. However, current evidence does not support the routine administration of recombinant Factor VIIa [13]. Erythromycin 125–250 mg IV is given at least 30 min prior to upper endoscopy to improve visualization. Nasogastric lavage can be performed prior to band ligation if needed and theoretically decreases the risk for HE by lowering ammonia production due to retained blood [4, 14].
Vasoactive medications terlipressin (not available in the USA) and somatostatin analogues (octreotide) are considered first-line therapy in acute variceal bleed to decrease portal pressures and induce splanchnic vasoconstriction; however, terlipressin is the only medication with a documented survival benefit [4, 15] (see Table 1 for details). These agents should be continued for at least 5 days, during which the risk of rebleeding is at its peak. Prophylactic intravenous antibiotics in the setting of variceal bleeding have been shown to decrease the risk of bacterial infections, including spontaneous bacterial peritonitis (SBP), and to improve survival [16, 17]; commonly used antibiotics include fluoroquinolones and third-generation cephalosporins (e.g., ceftriaxone). Short-term (7 days) course of norfloxacin 400 mg po bid should be given in any patient with cirrhosis and GI bleed especially if patient is allergic to penicillin [18]. If ceftriaxone 1 g IV bid is given initially, transition to norfloxacin if patient discharged early to complete 7-day course. In patients with advanced cirrhosis, ceftriaxone 1 g IV bid is preferred especially if there is known quinolone resistance in the center. Norfloxacin should be avoided if patient is already on flouroquinolones for SBP prophylaxis. Proton pump inhibitors (esomeprazole 40 mg or pantoprazole 40 mg or lansoprazole 30 mg or omeprazole 40 mg) should be administered intravenous to prevent stress ulcers and ulcers from band ligation of esophageal varices [19, 20] and switched to oral formulation when patient able to tolerate.
Endoscopic therapy for diagnosis and definitive therapy with esophagogastroduodenoscopy should be performed within 6–12 h of admission. Endoscopic variceal band ligation or sclerotherapy can be performed for bleeding esophageal varices. However, banding has been shown to be superior and safer to sclerotherapy in the acute control of esophageal variceal bleeding [18]. If esophageal variceal bleeding is not controlled with combined pharmacologic and endoscopic therapy, or if recurrence occurs early, then a transjugular intrahepatic portosystemic shunt (TIPS) may provide improved survival. Balloon tamponade with a Minnesota or Sengstaken-Blakemore tube is effective in temporary control of variceal bleeding and can be effective as a bridge to TIPS therapy in the setting of variceal bleeding that is refractory to endoscopic therapy. Complications from balloon tamponade include aspiration, esophageal perforation/necrosis, and migration [4, 21].
In setting of isolated gastric variceal bleeding, endoscopic cyanoacrylate (glue) injection is considered as an initial approach in many centers worldwide and in a few select centers in the USA with this expertise. Endoscopic cyanoacrylate (glue) injection achieves hemostasis in >90 % of cases, with rebleeding rates around 15 %. However, there is a 1–2 % risk of further bleeding, abdominal discomfort, and systemic embolization with glue therapy (splenic infarction, SVT, and intestinal infarction have also been reported) [22, 23, 24]. Given the absence of this endoscopic therapy in most US centers, TIPS is pursued early to achieve hemostasis.
Although TIPS is considered typically as salvage therapy for endoscopically refractory bleeding, early TIPS (<48 h from admission) in patients considered high risk for treatment failure, including Child–Pugh grade C or Child–Pugh grade B with active bleeding, was shown to improve survival compared to controls (39 vs. 14 %, p < 0.05) and decrease treatment failure. In addition, there were no differences in the subsequent development of HE, and durable results were seen in a post-trial surveillance study [25, 26].
Ascites and Spontaneous Bacterial Peritonitis (SBP)
Sodium retention and portal hypertension are the main causes for ascites in cirrhotic patients. In all ICU patients, early diagnostic paracentesis should be performed to assess for SBP. SBP is a known precipitant of hepatorenal syndrome (HRS), which is a cause of increased mortality in cirrhotic patients. Patients with tense ascites can develop elevated abdominal pressures and abdominal compartment syndrome, consequently leading to restrictive lung mechanics, hypotension, oliguria, and mesenteric ischemia [4, 27].Thus, a timely large-volume paracentesis (LVP), along with the administration of albumin 8 g/l of ascitic fluid removed, should be considered in this setting. Albumin administration prevents circulatory dysfunction with LVP.
SBP is defined as an ascitic fluid infection characterized by ascitic fluid absolute polymorphonuclear cell (PMN, also referred to as neutrophils) count ≥250 cells/mm3, positive culture results, and secondary causes of peritonitis (surgically treated cause) excluded [28]. Empiric antibiotic treatment should be initiated in patients whom the suspicion for SBP is high while awaiting results of the paracentesis. Current guidelines in this area need to be updated as more evidence has been published to guide antibiotic choice. In patients with a community acquired SBP, ceftriaxone 1 g IV bid or cefotaxime 2 g IV every 8 h for 5 days should be preferred. However, in cases with nosocomial acquired SBP, third-generation cephalosporins are avoided. Antibiotic choice should be guided by local microbial resistance patterns. Carbepenams like Meropenam 1 g every 8 h along with vancomycin for 5 days are preferred due to concern for ESBL (extended spectrum beta-lactamase) and gram-positive organism. If VRE is suspected, Linezolid 600 mg IV bid is preferred. In our experience in the setting of nosocomial SBP, a fungal etiology should be considered in addition to a bacterial process, especially if the patient is not responding to empiric antibacterial therapy. Once SBP is confirmed, IV albumin should be administered at a dose of 1.5 mg/kg at time of diagnosis and 1 mg/kg on the third day. This strategy was shown in a RCT to decrease risk of HRS and improve short-term survival from 29 to 10 % [29]. Sodium intake should be restricted to 2 g/day. If clinically feasible, combination diuretic therapy with spironolactone and furosemide in a ratio of 100 and 40 mg daily, respectively, should be given if urine sodium excretion is greater than 30 mmol/day. In patients with refractory ascites, TIPS is considered second-line treatment [27]. Peritoneal catheters should not be placed unless comfort care only is considered, as they carry a very high risk for infectious complications. More recently, an ALFA pump system was developed as a potential treatment option for refractory ascites, but was associated with many complications; therefore, further studies are needed before advocating this procedure [30].
With respect to the deleterious effects of concomitant medications in the setting of ascites and SBP, evidence has emerged regarding the potential negative effects of proton pump inhibitors (PPIs) and non-selective beta blockers. In a retrospective study, acid suppressive therapy was found to be associated with the development of SBP in cirrhotic patients with ascites. In addition, PPIs were associated with increased mortality following an episode of SBP, independent of the severity of the underlying liver disease [31].
Non-selective beta blocker therapy should perhaps be avoided in patient with refractory ascites, given the potential negative effects on systemic hemodynamics and renal function [32]. In addition, in the setting of SBP, non-selective beta blocker therapy has been found to increase hemodynamic compromise, time of hospitalization, risk for hepatorenal syndrome and acute kidney injury, and decreased transplant-free survival [33].
Sepsis and Cardiovascular Derangements
Cirrhotic patients are highly susceptible to infections due to immunologic deficits, decreased complement levels, impaired antigen-presenting ability, and impaired macrophage clearance of antibody-coated bacteria [34]. The most common infections occurring in cirrhotic patients are SBP, urinary tract infections, pneumonia, cellulitis, and bacteremia [4]. Bloodstream infections are independent predictors of ICU mortality in these patients [35]. Moreover, there have been increasing reports of multi-drug-resistant infections in cirrhotics due to widespread use of prophylactic antibiotics to prevent SBP. One study on potentially preventable nosocomial infections reported a 40 % case fatality rate associated with Clostridium difficile infection [36•]. The delay in diagnosis may be due to attributing the diarrhea to lactulose therapy.
Early goal-directed therapy (EGDT) in the treatment of sepsis is well established and continues to play a role in management of sepsis in cirrhosis.
However, no optimal endpoints have been studied for EGDT in cirrhotics, and guidelines established in general critical care have been extended to the management of critically ill cirrhotics [3]. It is important to understand that cirrhotic patients have an altered baseline hemodynamic profile characterized by a distributive hypotension-like pattern, with a low mean arterial pressure and a wide pulse pressure due to decreased systemic vascular resistance. This baseline physiology can be further exacerbated by a subsequent hypovolemic or septic hypotensive insult, such as a large-volume paracentesis or an infectious trigger, respectively.
Hemodynamic assessment in the critically ill cirrhotic is challenging. It is recognized in general critical care that traditional “static” indices of intravascular assessment, such as central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP), have their limitations, and this is particularly pertinent in the critically ill cirrhotic. Therefore, the use of “dynamic” indices of preload, such as stroke volume variation (SVV) and pulse pressure variation (PPV), should be utilized to guide therapy related to volume resuscitation and vasoactive agent support [37]. In the setting of shock, evaluation of global tissue ischemia is crucial in the critically ill cirrhotic, as it is a key development preceding end organ damage [3, 4, 38–41]. Therefore, the prompt initiation of EGDT in the septic cirrhotic patient is of paramount importance.
The approach to management includes a thorough assessment for the source of the infection, including a diagnostic paracentesis, urinary analysis, chest radiography, and blood and urine cultures prior to administering antibiotics. Broad-spectrum antibiotics should be administered based on local sensitivities [34]. Interestingly, the Cooperative Antimicrobial Therapy for Septic Shock research in critically ill cirrhotic patients found increased mortality with every hour in delay with antibiotic administration [42]. During the initiation of EGDT, it should be acknowledged that the baseline “normal” mean arterial pressure (MAP) maybe low, and therefore the goal of resuscitation should maybe aim for a MAP less than the conventional target MAP of 65 mmHg [34]. The risk of over resuscitation is that it could worsen portal pressures, leading to further systemic decompensation.
As in the management of septic shock in the non-cirrhotic, norepinephrine and low-dose vasopressin are typically the initial agents of choice for distributive shock. Dopamine is avoided as it can cause cardiac arrhythmias and vasodilation in the splanchnic circulation, thereby worsening portal hypertension [43]. In addition, there is some evidence that critically ill cirrhotics may have a high risk of relative adrenal insufficiency and, therefore, may benefit from stress dose steroids in the setting of shock that is refractory to vasoactive agent therapy [44]. From a cardiac perspective, there is evidence emerging that some cirrhotics may demonstrate derangements at a myocardial level, characterized by systolic and diastolic dysfunction, conductance system abnormalities, and a reduced response to beta agonist agents [45]. In these patients, there maybe a role for non-beta agonist therapy to provide inotropic support (e.g., milrinone) instead of dobutamine in the setting of a decreased ejection fraction (EF). Apart from attempting beta agonist therapy for systolic dysfunction and observing non-responsiveness, there is no diagnostic tool to a priori predict beta agonist non-responsivity. Therefore, a lack of response to beta agonist therapy should trigger a consideration for a non-beta agonist agent such as milrinone.
Coagulation Derangements
Coagulation abnormalities are common in cirrhotics due hepatic synthetic dysfunction, low platelets, enhanced fibrinolysis, and platelet dysfunction with coexisting renal failure. Conventional assays to determine coagulation status, such as prothrombin time (PT), have their limitations in the setting of cirrhosis since the net state of coagulopathy is unclear given the loss of both procoagulant and anticoagulant hepatic factors. Given the limitations of conventional testing, there may be a role for thromboelastography (TEG) to determine the coagulation status in a critically ill cirrhotic. In the absence of bleeding, an elevated prothrombin time (PT) should not be corrected since it does not necessarily reflect an increased risk of bleeding. In addition, the administration of fresh frozen plasma (FFP) can be associated with transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload, and transfusion reactions [46]. Vitamin K can be given to improve clotting factor synthesis, especially in the setting of cholestatic liver disease and malnutrition.
In patients with active bleeding, FFP should be administered with red blood cells in a ratio of 1:4 or 1:2 based on severity of bleeding. Cryoprecipitate should be given in the setting of a fibrinogen level <100 mg/dl, while platelets should be transfused to >50,000/mm3. Anti-fibrinolytic agents such as aminocaproic acid may be considered in the setting of bleeding that is refractory to the above interventions. Deep vein thrombosis (DVT) prophylaxis with mechanical compression devices should be provided, as venous thromboembolism is common in cirrhotic patients despite their apparent coagulopathy [1, 47–49].
Pulmonary Derangements
Mechanical Ventilation
Cirrhotic patients in the ICU often require mechanical ventilation (MV) due to significantly impaired gas exchange. A retrospective study in patients with cirrhosis requiring MV identified it to be a significant risk factor for mortality, with a MELD >21 being associated with a mortality ≥75 % at 1 year without liver transplantation [50]. Given their immunocompromised state, cirrhotic patients are at risk for developing acute respiratory distress syndrome (ARDS), which should be managed with the well-established strategy of low tidal volume ventilation (<6 ml/kg of ideal body weight) in order to minimize barotrauma and to optimize outcomes [3, 4].
In some patients, massive ascites can induce restrictive lung mechanics and therefore impair ventilation and oxygenation. A large-volume paracentesis (LVP) can significantly improve gas exchange in this situation. Similarly, a large hepatic hydrothorax can cause pulmonary and circulatory compromise. Thoracocentesis of approximately 2 l can help in those situations, and fluid should be sent for analysis to rule out spontaneous bacterial pleuritis. Large-bore chest tube placement is contraindicated as it can case hypovolemic shock, reexpansion pulmonary edema, and precipitate renal failure. In our experience, smaller-bore pleural drainage catheters (e.g., 10F biliary drainage catheters) with gravity drainage can facilitate the controlled removal of pleural fluid with stabilization of gas exchange without hemodynamic derangements [1, 4, 51]. TIPS may be beneficial in patients with refractory hydrothorax [4].
If sedation for mechanical ventilation is required, short-acting agents such as fentanyl and propofol can be utilized. Benzodiazepines should be avoided since they can worsen hepatic encephalopathy and increase the risk of delirium. Standard ventilator bundle and sedation vacation protocols should be performed with due diligence in order to minimize the duration of mechanical ventilation [1].
Hepatopulmonary Syndrome
Hypoxemia in a critically ill cirrhotic patient can be from various causes, including pneumonia, atelectasis, and effusions. However, hepatopulmonary syndrome (HPS) is an additional unique etiology of hypoxemia caused by diffusion-limited transfer of oxygen across the alveolar-capillary interface. HPS can present with platypnea, orthodeoxia, cyanosis, digital clubbing, shortness of breath, and hypoxemia. Diagnostic criteria include a PaO2 less than 70 mmHg on room air with an increased A-a gradient without CO2 retention [130]. In addition, a bubble echo or technetium Tc 99m macroaggregated albumin lung perfusion scan should be performed to establish the presence of intrapulmonary vasodilatation. In patients with HPS, the bubble echo demonstrates delayed bubbles in the left heart (within three to six beats after visualization in the right heart) due to the passage of the bubbles across the entire dilated pulmonary circulation. Orthotopic liver transplantation (OLT) offers the best survival, and these patients get MELD exception points for OLT for an arterial PaO2 ≤60 mmHg on room air [4, 51–53]. Typically, there is complete reversal of hypoxemia in weeks to months following OLT irrespective of the degree of pre-transplant hypoxemia.
Portopulmonary Hypertension
Portopulmonary hypertension (PPH) is a unique form of pulmonary arterial hypertension in the setting of cirrhosis. PPH has an estimated 5-year survival of 14 % without treatment. Dyspnea on exertion is the most common presenting symptom, but diagnosis is usually delayed. In later stages, patients may develop signs of right heart failure with orthopnea, fluid overload, chest pain, fatigue, or hemoptysis [54, 55]. PPH is suspected with an elevated estimated right ventricular systolic pressure (RVSP) on a transthoracic echocardiogram. A right heart catheterization is indicated with a RVSP >50 mmHg. Diagnostic criteria for PPH on heart right catheterization include a mean pulmonary artery pressure (mPAP) greater than 25 mmHg, normal pulmonary wedge pressure, and an elevated pulmonary vascular resistance greater than 125 dyne/s/cm5. Patients with a mPAP <35 mmHg are candidates for OLT, but a mPAP >35 mmHg is prohibitive for transplant given the high risk of acute right heart failure following reperfusion of the liver graft. Prostanoids, endothelin inhibitors, and phosphodiesterase inhibitors are different options that can be utilized in this patient population, with a goal to achieve an acceptable mPAP <35 mmHg to facilitate transplantation. No specific evidence-based guidelines have been established in this patient population, and therapy is based on the extrapolation of established protocols in other forms of pulmonary arterial hypertension. PPH usually persists after OLT with the necessity to continue pharmacologic therapy and does not exhibit the reversible characteristics that are observed in HPS [4, 51].
Renal Derangements
Acute kidney injury (AKI) in cirrhotics can be caused by various etiologies, including hypovolemia of any cause, hepatorenal syndrome, and medications. The incidence of AKI in cirrhotics admitted to the ICU is 40–60 % and is considered to be a poor prognostic sign as it is associated with an increased risk for HE and mortality. In a recent study, 30-day mortality was 10-fold higher among those with irreversible AKI than those without AKI [56••].
Traditional diagnostic criteria for HRS includes cirrhosis with ascites, serum creatinine >1.5 mg/dl, diuretic withdrawal and volume expansion with albumin for 2 days with no improvement of creatinine, absence of shock or nephrotoxic medications, and ruling out parenchymal kidney disease [4, 27]. However, in light of the new definitions of acute kidney injury (AKI) in the critical care literature, there is currently an impetus for refining HRS criteria. A recent consensus conference defined cirrhosis-associated AKI as “an increase in serum creatinine of more than 50 % from the stable baseline value or by 0.3 mg/dl (27 mmol/l) in less than 48 h.” This definition accurately predicted 30-day mortality, length of hospital stay, and organ failure in a study [56••].
Type 1 HRS is defined as acute doubling of initial serum creatinine to greater than 2.5 mg/dl or a 50 % reduction of the initial 24-h creatinine clearance to a level less than 20 ml/min in less than 2 weeks. Type II HRS is associated with diuretic-resistant ascites and serum creatinine ranging from 1.5 to 2.5 mg/dl [27].
Management of AKI in the cirrhotic includes prompt identification and treatment of the cause. Antibiotics should be administered early if an infectious septic trigger is suspected, especially SBP. However, in a recent study, type 1 HRS associated with infections was found not to be reversible with treatment of infection only and implied a poor prognosis with a 3-month survival of only 27 % [57••].
In the USA, treatment for type 1 HRS includes triple therapy with octreotide (continuous intravenous infusion 50 μg/h or subcutaneously 100 to 200 μg three times daily), midodrine (starting at 7.5 mg and increasing the dose at 8-h intervals up to a maximum of 15 mg by mouth three times daily with the goal of raising the mean arterial pressure by 10 mmHg) or norepinephrine (0.5 to 3 mg/h) with the goal of raising the mean arterial pressure by 10 mmHg and albumin (1 g/kg daily max 100 g daily) for 2 days followed by 25 g daily, which can improve renal function and hemodynamics by mitigating splanchnic and systemic vasodilatation. Norepinephrine is preferred in the ICU setting, with a goal to titrate the norepinephrine to achieve an increase in the MAP of 10 mmHg [27]. In Europe, albumin (1 g/kg at the start of treatment, followed by 20–40 g/day) along with terlipressin 1–2 mg/q4–6hours for a minimum of 72 h is used, which has shown improvement in renal function but no survival benefit [58]. Renal replacement therapy (RRT) should be initiated in a timely manner if indications for this intervention are present such as hyperkalemia and metabolic acidosis. Patients with RRT greater than 6 weeks perform better from a renal standpoint with a combined/liver kidney transplant [4], and therefore, most transplant centers follow this policy. Albumin dialysis with MARS or Prometheus systems has not demonstrated any beneficial effects on the reversal of HRS in the few trials performed to date [2, 3, 59].
Electrolyte Derangements
Hyponatremia in cirrhotics is defined as a level less than 130 mEq/l and affects 30 % of cirrhotic patients. Studies have shown hyponatremia to be an important predictor of health-related quality of life independent of HE, besides a predictor of poor morbidity and mortality. However, correction of hyponatremia has not shown any survival benefit [60–62]. Hyponatremia can be either hypervolemic in nature (most common), due to expanded extracellular fluid volume and impaired renal tubular water excretion, or hypovolemic from fluid losses (commonly from diuretics) [63]. Work-up for the specific type of hyponatremia should be performed, as treatment differs. Volume repletion should be done for hypovolemic hyponatremia. In the setting of hypervolemic hyponatremia, fluid restriction is the mainstay of treatment once the serum sodium approaches 120–125 mEq/l. With severe or symptomatic hyponatremia associated with a serum sodium ≤120 mEq/l, hypertonic saline can be considered, but could worsen ascites and edema [27, 61]. There is some evidence for the benefits of albumin infusion from two small studies to correct severe hyponatremia [64]. Another pharmacologic option is tolvaptan, which is a selective V2 receptor antagonist that can prevent the absorption of free water in the collecting tubule and therefore causes free water elimination. Utility of tolvaptan is limited due to a black box warning by FDA for hepatotoxicity and removal of indication for use in cirrhotics (http://www.fda.gov/downloads/Drugs/DrugSafety/UCM350084.pdf).
Hypokalemia and hypomagnesemia should be promptly corrected as they can precipitate HE. If hyperchloremic acidosis is present, chloride-containing fluids should be avoided. With respect to acid base derangements, the critically ill cirrhotic is susceptible to the development of an anion gap metabolic acidosis due to the absence of hepatic lactate clearance. In addition, in the presence of concomitant AKI, the patient should be closely monitored for a rapidly progressive metabolic acidosis due to hepatic and renal dysfunction, with a consideration for early RRT [65].
Acute on Chronic Liver Failure
Acute on chronic liver failure (ACLF) is a newly defined distinct entity compared to decompensated cirrhosis. ACLF is characterized by an acute deterioration of liver function and often results in multi-organ system failure. This entity does not follow the usual sequence of compensated to decompensated liver disease. ACLF involves multi-organ system dysfunction in addition to hepatic dysfunction, and the prognosis worsens based on the number of extrahepatic organs involved. In a recent large multicenter European study, investigators developed a more evidence-based definition based on the presence of three major characteristics: acute decompensation, organ failure [predefined by the Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score], and a high 28-day mortality rate [59]. The most common triggers for ACLF in cirrhotic patients include infections, acute alcoholic hepatitis, viral hepatitis (A, B, E, B, and D coinfection), portal vein thrombosis, drug hepatotoxicity, ischemia of any cause, and GI bleeding [66].
Despite variable etiologies for ACLF, infections play a key role in the development of ACLF. Cirrhotic patients are more prone to infections compared to the general population due to immune dysfunction, and there is an increased risk of infection with multi-drug-resistant organisms, which portends increased mortality. The inflammatory response and cytokine storm evoked from infections plays a critical role in the development of ACLF and end-organ dysfunction. Early identification with a high index of suspicion for an infectious etiology, followed by appropriate antibiotic coverage, can prevent end organ damage [36•, 67, 68].
Nutritional Support
Malnutrition has been associated with a decreased quality of life, survival, and development of complications in cirrhosis [69]. There is no role for protein restriction; protein (0.8–1.2 g/kg/day) should be provided and adjusted based on the presence of sepsis or renal replacement therapy to prevent negative nitrogen balance. Due to impaired gluconeogenesis, cirrhotic patients are at risk for hypoglycemia, and the caloric requirements should be monitored closely. Thiamine should be replaced intravenously for 3–5 days [1, 70]. Enteral nutrition is preferred to parenteral nutrition in order to facilitate gut mucosal integrity. If there is a contraindication to enteral nutrition, parenteral nutrition can be cautiously administered, with close monitoring for infectious complications.
Role of Extracorporeal Liver Support Systems
Artificial liver support systems have been evaluated for various indications in cirrhosis, including HE, ACLF, and HRS. The Molecular Adsorbent Recirculating System (MARS) is FDA approved for the treatment of advanced hepatic encephalopathy in cirrhosis based on the demonstration of its efficacy for this indication in a randomized control trial [8]. However, other recent trials comparing extracorporeal albumin dialysis systems have not demonstrated a mortality benefit compared to standard medical therapy [59, 71]. Larger prospective studies are awaited to further examine the potential benefit of extracorporeal liver support systems in the setting of decompensated cirrhosis and ACLF.
Prognosis of Cirrhotic Patients Admitted to the ICU
The MELD and CPT scores have been used to estimate survival in cirrhotic patients. However, these scores do not accurately predict survival in the ICU for these patients since these scores do not account for multi-organ system dysfunction and failure. General ICU scores like the APACHE II, SOFA, and SAPS II are potentially better scoring systems to assess prognosis in the critically ill cirrhotic patient. More recently, the CLIF-SOFA has been proposed as a score that is more specific for the patient with ACLF and has been shown to be a good predictor of survival for ACLF [59]. In addition to the above scoring systems, an indication for ICU care itself predicts a higher mortality, with sepsis or multi-organ system dysfunction predicting worse outcomes compared to HE or GI bleeding [72, 73, 74••].
Conclusions
The critically ill cirrhotic patient presents a unique set of management challenges, with potential derangements in multiple organ systems. Successful resuscitation and stabilization of these patients is dependent upon a multi-professional approach to care delivery and, in particular, close collaboration between the hepatologist and intensivist.
Abbreviations
- AMS:
-
Altered mental status
- HE:
-
Hepatic encephalopathy
- SBP:
-
Spontaneous bacterial peritonitis
- HRS:
-
Hepatorenal syndrome
- HPS:
-
Hepatopulmonary syndrome
- PPH:
-
Portopulmonary hypertension
- ACLF:
-
Acute on chronic renal failure
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Olson JC et al. Intensive care of the patient with cirrhosis. Hepatology. 2011;54(5):1864–72.
Karvellas CJ, Bagshaw SM. Advances in management and prognostication in critically ill cirrhotic patients. Curr Opin Crit Care. 2014;20(2):210–7.
Gines P et al. Management of critically-ill cirrhotic patients. J Hepatol. 2012;56 Suppl 1:S13–24.
Ford RM, Sakaria SS, Subramanian RM. Critical care management of patients before liver transplantation. Transplant Rev (Orlando). 2010;24(4):190–206.
Mullen KD et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12(8):1390–1397 e2.
Bass NM et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–81.
Mas A et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51–8.
Hassanein TI et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology. 2007;46(6):1853–62.
Laleman W et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–57.
Garcia-Tsao G et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5(3):419–24.
Carbonell N et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40(3):652–9.
Villanueva C et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
Bosch J et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized, controlled trial. Hepatology. 2008;47(5):1604–14.
Theivanayagam S et al. Administration of erythromycin before endoscopy in upper gastrointestinal bleeding: a meta-analysis of randomized controlled trials. Saudi J Gastroenterol. 2013;19(5):205–10.
Levacher S et al. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet. 1995;346(8979):865–8.
Bernard B et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29(6):1655–61.
Soares-Weiser K et al. Antibiotic prophylaxis of bacterial infections in cirrhotic inpatients: a meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2003;38(2):193–200.
Garcia-Tsao G et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922–38.
Shaheen NJ et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology. 2005;41(3):588–94.
Boo GB et al. The effect of proton pump inhibitor on healing of post-esophageal variceal ligation ulcers. Korean J Gastroenterol. 2008;51(4):232–40.
de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43(1):167–76.
Romero-Castro R et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc. 2013;78(5):711–21.
Procaccini NJ et al. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009;70(5):881–7.
Crisan D, Tantau M, Tantau A. Endoscopic management of bleeding gastric varices—an updated overview. Curr Gastroenterol Rep. 2014;16(10):413.
Garcia-Pagan JC et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58(1):45–50.
Garcia-Pagan JC et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–9.
Runyon BA, Committee APG. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–107.
Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27(4):669–74. quiz 675–6.
Pleguezuelo M et al. Diagnosis and management of bacterial infections in decompensated cirrhosis. World J Hepatol. 2013;5(1):16–25.
Bellot P et al. Automated low flow pump system for the treatment of refractory ascites: a multi-center safety and efficacy study. J Hepatol. 2013;58(5):922–7.
Kwon JH et al. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2014;29(4):775–81.
Serste T et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52(3):1017–22.
Mandorfer M et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146(7):1680–90 e1.
Gustot T et al. Severe sepsis in cirrhosis. Hepatology. 2009;50(6):2022–33.
Karvellas CJ et al. Bacteremia, acute physiology and chronic health evaluation II and modified end stage liver disease are independent predictors of mortality in critically ill nontransplanted patients with acute on chronic liver failure. Crit Care Med. 2010;38(1):121–6.
Bajaj JS et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56(6):2328–35. Prospective cohort study showed potentially preventable second infections are predictors of mortality independent of severity of cirrhosis.
Monnet X, Teboul J-L. Prediction of fluid responsiveness in patients with shock. Clin Pulm Med. 2014;21(6):282–7.
Rivers E et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77.
Early Goal-Directed Therapy Collaborative Group of Zhejiang, P. The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/septic shock: a multi-center, prospective, randomized, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2010;22(6):331–4.
Moreau R et al. Abnormal tissue oxygenation in patients with cirrhosis and liver failure. J Hepatol. 1988;7(1):98–105.
Dellinger RP et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
Arabi YM et al. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56(6):2305–15.
De Backer D et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–89.
Acevedo J et al. Relative adrenal insufficiency in decompensated cirrhosis: relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology. 2013;58(5):1757–65.
Moreau R et al. Septic shock in patients with cirrhosis: hemodynamic and metabolic characteristics and intensive care unit outcome. Crit Care Med. 1992;20(6):746–50.
Kor DJ, Gajic O. Blood product transfusion in the critical care setting. Curr Opin Crit Care. 2010;16(4):309–16.
Bianchini M, De Pietri L, Villa E. Coagulopathy in liver diseases: complication or therapy? Dig Dis. 2014;32(5):609–14.
Intagliata NM et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014;34(1):26–32.
Yang ZJ et al. Venous thromboembolism in cirrhosis. Clin Appl Thromb Hemost. 2014;20(2):169–78.
Levesque E et al. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60(3):570–8.
Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59(4):1627–37.
Sharma P, Rakela J. Management of pre-liver transplantation patient—part 2. Liver Transpl. 2005;11(3):249–60.
Ramsay MA. Portopulmonary hypertension and hepatopulmonary syndrome, and liver transplantation. Int Anesthesiol Clin. 2006;44(3):69–82.
Talwalkar JA et al. Prevalence of spontaneous portosystemic shunts in patients with portopulmonary hypertension and effect on treatment. Gastroenterology. 2011;141(5):1673–9.
Colle IO et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37(2):401–9.
Wong F et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(6):1280–8 e1. Consensus conference proposed definition for cirrhosis-associated acute kidney injury (AKI).
Barreto R et al. Type-1 hepatorenal syndrome associated with infections in cirrhosis: natural history, outcome of kidney function, and survival. Hepatology. 2014;59(4):1505–13. This prospective study shows two thirds of patients with HRS-1 associated with infections is irreversible.
European Association for the Study of the, L, European Association for the Study of the, L. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417.
Banares R et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57(3):1153–62.
Kwo PY. Management of hyponatremia in clinical hepatology practice. Curr Gastroenterol Rep. 2014;16(5):382.
Solà E, Graupera I, Ginès P. From refractory ascites to dilutional hyponatremia and hepatorenal syndrome: current options for treatment. Curr Hepatol Rep. 2014;1–9.
Ahluwalia V et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J Hepatol. 2013;59(3):467–73.
Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48(3):1002–10.
Jalan R et al. Albumin infusion for severe hyponatremia in patients with refractory ascites: a randomized clinical trial. J Hepatol. 2007;46:S95.
Davenport A. Acute renal replacement therapy. In: Harber M, editor. Practical nephrology. London: Springer; 2014. p. 75–90.
Asrani SK, O'Leary JG. Acute-on-chronic liver failure. Clin Liver Dis. 2014;18(3):561–74.
Jalan R et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60(6):1310–24.
Fernandez J et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55(5):1551–61.
Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20(25):8061–71.
Butterworth RF. Thiamine deficiency-related brain dysfunction in chronic liver failure. Metab Brain Dis. 2009;24(1):189–96.
Kribben A et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142(4):782–789 e3.
Saliba F et al. Cirrhotic patients in the ICU: prognostic markers and outcome. Curr Opin Crit Care. 2013;19(2):154–60.
Cholongitas E et al. Prognostic models in cirrhotics admitted to intensive care units better predict outcome when assessed at 48 h after admission. J Gastroenterol Hepatol. 2008;23(8pt1):1223–7.
Moreau R., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–37. 1437 e1-9. Data from cirrhotics with acute decompensation was utilized to define Acute on chronic liver failure as a distinct entity.
Compliance with Ethics Guidelines
Conflict of Interest
Sunil Dacha declares no conflict of interest.
Ram M. Subramanian is a consultant for Gambro-Baxter, Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Management of the Cirrhotic Patient
Rights and permissions
About this article
Cite this article
Dacha, S., Subramanian, R.M. Critical Care Management in Cirrhosis. Curr Hepatology Rep 14, 60–68 (2015). https://doi.org/10.1007/s11901-015-0255-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-015-0255-9