Abstract
Purpose of Review
Type 1 diabetes (T1D) is an autoimmune disease in which the immune cells selectively destroy the pancreatic beta (β) cells and results in the deficiency of insulin production. The optimal treatment strategy for T1D should be preventing of β-cell destruction in the pancreas. The purpose of this review is to discuss the immunological therapeutic mechanisms that will help to understand the development and control of β-cell destruction. The review also presents a novel method for development of autoantigen (Ag)-specific regulatory T cells (Tregs) for T1D immunotherapy.
Recent Findings
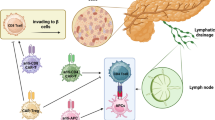
Pancreatic-resident Tregs have the ability to dramatically suppress hyperactive immune cells. Islet cell transplantation is another attractive approach to replace the failed β cells. Due to the limited source of islet cells, research is going on in the use of animal cells and adult stem cells that may be derived from the patient’s own body to produce β cells for transplantation.
Summary
The mechanism behind the pancreatic β-cell destruction is largely unknown. In this review, a novel approach for the generation of tissue-associated Tregs from stem cells is considered. The stem cell-derived tissue-associated Tregs have the ability to home to the damaged pancreas to prevent the destruction. The review also provides new insights on the mechanism on how these suppressive immune cells protect the pancreas from the destruction of autoimmune cells. A novel method to develop functional auto Ag-specific Tregs that are derived from induced pluripotent stem cells (iPSCs), i.e., iPSC-Tregs, is discussed. Adoptive transfer of the iPSC-Tregs can substantially suppress T1D development in a murine model.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, Condon JE, et al. 2017 national standards for diabetes self-management education and support. Diabetes Educ. 2019;45(1):34–49.
Rariden C. Prediabetes: a wake-up call. Nursing. 2019;49(4):38–44.
Egro FM. Why is type 1 diabetes increasing? J Mol Endocrinol. 2013;51(1):R1–13.
James EA, Pietropaolo M, Mamula MJ. Immune recognition of beta-cells: neoepitopes as key players in the loss of tolerance. Diabetes. 2018;67(6):1035–42.
Michels AW, Gottlieb PA. Learning from past failures of oral insulin trials. Diabetes. 2018;67(7):1211–5.
Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–47.
• Spence A, Purtha W, Tam J, Dong S, Kim Y, Ju CH, et al. Revealing the specificity of regulatory T cells in murine autoimmune diabetes. Proc Natl Acad Sci U S A. 2018;115(20):5265–70. Findings from this study provide a glimpse into the specificities of Tregs in a natural repertoire that are crucial for opposing the progression of autoimmune diabetes.
• Yu H, Gagliani N, Ishigame H, Huber S, Zhu S, Esplugues E, et al. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc Natl Acad Sci U S A. 2017;114(39):10443–8. Findings from this study suggest that modulating gut-associated lymphoid tissue to boost Tr1 cells may be important in type 1 diabetes management.
Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28(6):870–80.
Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45.
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86.
Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205(9):1975–81.
Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Front Oncol. 2014;4:209.
•• Haque M, Lei F, Xiong X, Das JK, Ren X, Fang D, et al. Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes. JCI Insight. 2019;4(7). Findings from this study suggest that the stemcell-derived tissue-associated Tregs can robustly accumulate in the diabetic pancreas, and, through downregulating the expression of ICAM-1 in the local inflamed tissues and inhibiting the production of proinflammatory cytokine IFN-γ, suppress the migration and activity of the pathogenic immune cells that cause T1D.
Lei F, Haque R, Weiler L, Vrana KE, Song J. T lineage differentiation from induced pluripotent stem cells. Cell Immunol. 2009;260(1):1–5.
Lei F, Zhao B, Haque R, Xiong X, Budgeon L, Christensen ND, et al. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011;71(14):4742–7.
Lei F, Haque R, Xiong X, Song J. Directed differentiation of induced pluripotent stem cells towards T lymphocytes. J Vis Exp. 2012;63:e3986.
Haque R, Lei F, Xiong X, Bian Y, Zhao B, Wu Y, et al. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol. 2012;189(3):1228–36.
Haque M, Song J, Fino K, Sandhu P, Song X, Lei F, et al. Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity. Sci Rep. 2016;6:20588.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699–702.
• Lopez-Yrigoyen M, Yang CT, Fidanza A, Cassetta L, Taylor AH, McCahill A, et al. Genetic programming of macrophages generates an in vitro model for the human erythroid island niche. Nat Commun. 2019;10(1):881. Findings from this study suggest establishment of an in vitro system to model the human EI niche using macrophages that are derived from human iPSCs, and are also genetically programmed to an EI-like phenotype by inducible activation of the transcription factor, KLF1.
• Minagawa A, Yoshikawa T, Yasukawa M, Hotta A, Kunitomo M, Iriguchi S, et al. Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell Stem Cell. 2018;23(6):850–8 e4. Findings from this study suggest that enhancing TCR stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy.
Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29.
Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8(2):274–84.
Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–75.
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–53.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza I, Vassena R, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5(4):353–7.
Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283(46):31601–7.
• Enderami SE, Mortazavi Y, Soleimani M, Nadri S, Biglari A, Mansour RN. Generation of insulin-producing cells from human-induced pluripotent stem cells using a stepwise differentiation protocol optimized with platelet-rich plasma. J Cell Physiol. 2017;232(10):2878–86. Findings from this study suggest a new approach to investigate the role of PRP in pancreatic differentiation protocols and enhance the feasibility of using patient-specific iPSCs and autologous PRP for future beta cells replacement therapies for T1DM.
Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106(37):15768–73.
•• Millman JR, Xie C, Van Dervort A, Gurtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463 Findings from this study suggest that T1D SC-β cells could potentially be used for the treatment of diabetes, drug screening and the study of β-cell biology.
Martin S, Pawlowski B, Greulich B, Ziegler AG, Mandrup-Poulsen T, Mahon J. Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care. 1992;15(1):66–74.
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52.
Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53(4):614–23.
Gitelman SE, Gottlieb PA, Felner EI, Willi SM, Fisher LK, Moran A, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59(6):1153–61.
Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905–15.
Takemoto N, Konagaya S, Kuwabara R, Iwata H. Coaggregates of regulatory T cells and islet cells allow long-term graft survival in liver without immunosuppression. Transplantation. 2015;99(5):942–7.
Duggleby R, Danby RD, Madrigal JA, Saudemont A. Clinical grade regulatory CD4(+) T cells (Tregs): moving toward cellular-based immunomodulatory therapies. Front Immunol. 2018;9:252.
Marek-Trzonkowska N, Mysliwiec M, Iwaszkiewicz-Grzes D, Gliwinski M, Derkowska I, Zalinska M, et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med. 2016;14(1):332.
Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189.
Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164(1):183–90.
• Boroughs AC, Larson RC, Choi BD, Bouffard AA, Riley LS, Schiferle E, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight. 2019;5. Findings from this study suggest the use of CD28 based CAR-Tregs for tissue specific immune suppression in the clinic.
• Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol. 2018;30(10):445–54. Findings from this study suggest the new therapies that can reverse immune suppression in the TME and promote anti-tumorimmunity.
Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175(7):4180–3.
• Yoon J, Schmidt A, Zhang AH, Konigs C, Kim YC, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129(2):238–45. Findings from this study suggest that CAR-transduced Tregs are a promising approach for future tolerogenic treatment of hemophilia A patients with inhibitors.
Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12.
• Semitekolou M, Morianos I, Banos A, Konstantopoulos D, Adamou-Tzani M, Sparwasser T, et al. Dendritic cells conditioned by activin A-induced regulatory T cells exhibit enhanced tolerogenic properties and protect against experimental asthma. J Allergy Clin Immunol. 2018;141(2):671–84 e7. Findings from this study suggest that Act-A-iTreg cells instruct the generation of a highly effective immunoregulatory circuit encompassing tolerogenic DCs and forkhead box P3 + Treg cells that could be targeted for the design of novel immunotherapies for allergic disorders.
Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104(49):19446–51.
•• Guipouy D, Gertner-Dardenne J, Pfajfer L, German Y, Belmonte N, Dupre L. Granulysin- and granzyme-dependent elimination of myeloid cells by therapeutic ova-specific type 1 regulatory T cells. Int Immunol. 2019;31(4):239–50. Findings from this study suggest that ova-Tr1 cells are endowed with a sustained cytotoxic activity that relies on a unique combination of granulysin and granzymes and that preferentially eliminates myeloid target cells in a TCR-independent manner.
Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–40.
Aiello LP, Group DER. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):17–23.
Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52(7):2212–21.
•• Sun G, Hou Y, Gong W, Liu S, Li J, Yuan Y, et al. Adoptive induced antigen-specific treg cells reverse inflammation in collagen-induced arthritis mouse model. Inflammation. 2018;41(2):485–95. Findings from this study suggest that adoptive induced antigen-specific Treg cells may have clinical applications for treatment of autoimmunity, including RA and other autoimmune disorders.
Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169(9):4712–6.
• Wilkinson DS, Ghosh D, Nickle RA, Moorman CD, Mannie MD. Partial CD25 antagonism enables dominance of antigen-inducible CD25(high) FOXP3(+) regulatory T cells as a basis for a regulatory T cell-based adoptive immunotherapy. Front Immunol. 2017;8:1782. Findings from this study suggest that low IL-2 concentrations coupled with high PC61 concentrations constrained IL-2 signaling to a low-intensity range that enabled dominant stable outgrowth of suppressive CD25 high FOXP3 + Tregs. The ability to indefinitely expand stable Treg lines will provide insight into FOXP3 + Treg physiology and will be foundational for Treg-based immunotherapy.
Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177(3):1451–9.
• Yang D, Tian Z, Zhang M, Yang W, Tang J, Wu Y, et al. NKG2D(+)CD4(+) T cells kill regulatory T cells in a NKG2D-NKG2D ligand-dependent manner in systemic lupus erythematosus. Sci Rep. 2017;7(1):1288 Findings from this study suggest that NKG2D +CD4 + T cells are involved in the pathogenesis of SLE by killing Treg cells in a NKG2D-NKG2DL-dependentmanner. Targeting the NKG2D-NKG2DL interaction might be a potential therapeutic strategy by which Treg cells can be protected from cytolysis in SLE patients.
Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69(20):7895–8.
Hutmacher C, Gonzalo Nunez N, Liuzzi AR, Becher B, Neri D. Targeted delivery of IL2 to the tumor stroma potentiates the action of immune checkpoint inhibitors by preferential activation of NK and CD8(+) T cells. Cancer Immunol Res. 2019;7(4):572–83.
Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7(1):83–92.
• Seitz C, Liu S, Klocke K, Joly AL, Czarnewski PV, Tibbitt CA, et al. Multi-faceted inhibition of dendritic cell function by CD4(+)Foxp3(+) regulatory T cells. J Autoimmun. 2019;98:86–94. Findings from this study suggest that Treg cells induced a specific immunosuppressive program in DCs.
Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173(5):2942–51.
Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ Treg cells impinge on autoimmune diabetes. J Exp Med. 2005;202(10):1387–97.
•• Zou J, Gao X, Liu T, Liang R, Liu Y, Wang G, et al. Ethylenecarbodiimide-fixed splenocytes carrying whole islet antigens decrease the incidence of diabetes in NOD mice via down-regulation of effector memory T cells and autoantibodies. Endocr J. 2018;65(9):943–52. Findings from this study suggest that ECDI-fixed splenocytes carrying whole isletantigens effectively prevented the onset of T1DM in NOD mice, via suppressing the production of autoantibodies and inducing anergy of autoreactive T cells.
Roncarolo MG, Battaglia M, Gregori S. The role of interleukin 10 in the control of autoimmunity. J Autoimmun. 2003;20(4):269–72.
•• Abdel-Latif M, Abdel-Moneim AA, El-Hefnawy MH, Khalil RG. Comparative and correlative assessments of cytokine, complement and antibody patterns in paediatric type 1 diabetes. Clin Exp Immunol. 2017;190(1):110–21. Findings from this study suggest that IFN-γ, IL-12 and IL-17 played an essential role in exacerbating EV +-T1D, while C3d, sC5b-9, IL-10 and -20 displayed distinct patterns.
Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A. 2004;101(13):4572–7.
• Kuhn C, Rezende RM, da Cunha AP, Valette F, Quintana FJ, Chatenoud L, et al. Mucosal administration of CD3-specific monoclonal antibody inhibits diabetes in NOD mice and in a preclinical mouse model transgenic for the CD3 epsilon chain. J Autoimmun. 2017;76:115–22. Findings from this study suggest that oral CD3-specific mAb has the potential for treating autoimmune diabetes in humans.
Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, et al. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes. 2006;55(6):1571–80.
Dai B, Wang P. In vitro differentiation of adult bone marrow progenitors into antigen-specific CD4 helper T cells using engineered stromal cells expressing a notch ligand and a major histocompatibility complex class II protein. Stem Cells Dev. 2009;18(2):235–45.
• Haque R, Song J, Haque M, Lei F, Sandhu P, Ni B, et al. c-Myc-induced survivin is essential for promoting the notch-dependent T cell differentiation from hematopoietic stem cells. Genes (Basel). 2017;8(3). Findings from this study suggest both c-Myc and survivin as important mediators of the Notch signaling-regulated differentiation of T lymphocytes from hematopoietic stem cells.
Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329(5999):1667–71.
Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184(3):923–30.
Guarantor’s Statement
J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by the National Institutes of Health grants R01AI121180, R01CA221867, and R21AI109239 and by the American Diabetes Association (1-16-IBS-281) to J.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mohammad Haque, Jugal Kishore Das, Xiaofang Xiong, and Jianxun Song declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Immunology, Transplantation, and Regenerative Medicine
Rights and permissions
About this article
Cite this article
Haque, M., Das, J.K., Xiong, X. et al. Targeting Stem Cell-Derived Tissue-Associated Regulatory T Cells for Type 1 Diabetes Immunotherapy. Curr Diab Rep 19, 89 (2019). https://doi.org/10.1007/s11892-019-1213-7
Published:
DOI: https://doi.org/10.1007/s11892-019-1213-7