Abstract
Purpose of Review
The goal of this review is to describe diabetes within a population health improvement framework and to review the evidence for a diabetes population health continuum of intervention approaches, including diabetes prevention and chronic and acute diabetes management, to improve clinical and economic outcomes.
Recent Findings
Recent studies have shown that compared to usual care, lifestyle interventions in prediabetes lower diabetes risk at the population-level and that group-based programs have low incremental medial cost effectiveness ratio for health systems. Effective outpatient interventions that improve diabetes control and process outcomes are multi-level, targeting the patient, provider, and healthcare system simultaneously and integrate community health workers as a liaison between the patient and community-based healthcare resources. A multi-faceted approach to diabetes management is also effective in the inpatient setting. Interventions shown to promote safe and effective glycemic control and use of evidence-based glucose management practices include provider reminder and clinical decision support systems, automated computer order entry, provider education, and organizational change.
Summary
Future studies should examine the cost-effectiveness of multi-faceted outpatient and inpatient diabetes management programs to determine the best financial models for incorporating them into diabetes population health strategies.
Similar content being viewed by others
Introduction
Diabetes population trends, health outcomes, and healthcare costs make it a priority condition for population health improvement in the USA. An estimated 9.1% of the overall US population has diagnosed diabetes, 5.2% has undiagnosed diabetes, and an additional 38.0% has prediabetes [1•]. Diabetes is the sixth leading cause of disability in the USA [2] and is the seventh leading cause of death, with a 2014 age-adjusted mortality rate of 20.9 per 100,000 population [3]. In the adult US population aged 20 years and older, diabetes ranks highest among all disease categories in healthcare spending, with an estimated $101.4 billion in healthcare spending in 2013 [4]. As a disease of health inequities, racial and ethnic minority groups and persons with lower socioeconomic status experience higher diabetes prevalence, morbidity, and mortality rates [1•, 5, 6]. Over the past decade, evidence has grown for opportunities to impact diabetes and its outcomes across population risk strata and the intervention continuum inclusive of primary prevention, secondary prevention, and tertiary prevention. In this paper, we describe diabetes within a population health improvement framework, review evidence for a diabetes population health continuum of intervention approaches to improve clinical and economic outcomes, and review the intervention continuum within the context of diabetes standards of care and policy advancement.
Diabetes and Population Health
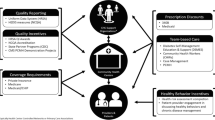
Population health has emerged as a framework to guide comprehensive interventions and policies for improving prevention, health promotion and healthcare outcomes, and addressing the determinants of health that contribute to health inequities [7, 8]. Population health is defined as “the health of a population as measured by health status indicators and as influenced by social, economic, and physical environments; personal health practices; individual capacity and coping skills; human biology; early childhood development; and health services” [9]. Consequently, population health broadens health improvement beyond traditional boundaries of medical care or public health and necessitates community and multi-sector partnerships for intervention implementation outside of healthcare settings, and targeted interventions within the healthcare setting [7, 8]. Population health methods include use of population assessment, risk stratification, targeted interventions to provide population subgroups in different risk strata appropriate and quality care in the right settings, and data to determine outcomes. Figure 1 presents a model for diabetes population health improvement incorporating these concepts.
Within healthcare settings and organizations, national diabetes quality measures are applied to populations with diabetes. The current National Quality Forum ambulatory diabetes metrics that are considered for Healthcare Effectiveness Data and Information Set (HEDIS) accreditation are summarized in Table 1. In our current healthcare model, Accountable Care Organizations are being incentivized for achieving diabetes care metrics in the patient populations in their catchment area, which has required them to develop effective healthcare delivery models that impact not only just individual patients but also the patient population as a whole. In contrast, despite the high costs of acute care of diabetes in the hospital setting, there are no uniformly endorsed inpatient glycemic quality metrics. As we recently reviewed, several professional societies have published guidelines for inpatient glycemic targets, process measures, and pharmacologic management [10]. Tables 2 and 3 summarize the current recommendations from several professional societies as well as the proposed Center for Medicare Services (CMS)/National Quality Forum (NQF) metrics. As seen in Table 3, there are notable differences in the definitions of the inpatient glucometrics, particularly with respect to the patient populations included in the denominator. For the Society of Hospital Medicine (SHM) [14] and Yale [15] metrics, all blood glucose (BG) data, including those obtained from patients who may not have received any glucose-lowering medications, are included in the metrics for normoglycemia, hyperglycemia, and hypoglycemia. The SHM and Yale groups have proposed definitions of normoglycemia that consist of either the percent of all BGs within a target range (70–179 mg/dl for SHM and 70–149 mg/dl for Yale) or the percent of patient days or patient stays in which all BG readings were within the defined target range. CMS has adopted publically endorsed NQF metrics for hypoglycemia and hyperglycemia, but has not proposed a metric for normoglycemia [16]. There is variability with respect to the hyperglycemic metric, with some definitions using a mean BG threshold (≥180 mg/dl) while others use a certain frequency of individual BG readings above a threshold (e.g., any BG >299 mg/dl or 2 or more BG readings >200 mg/dl). Finally, only SHM provides metrics for hypoglycemia management (i.e., time to resolution or time to repeat BG check) [14]. The benchmarking of this metric consists of ranking hospitals against others for performance (i.e., lower response time is better). Since there are agreed upon metrics for other aspects of diabetes population health, including diabetes prevention and ambulatory diabetes management, we will highlight and summarize effective interventions around these metrics, in addition to summarizing the available literature on effective inpatient interventions to improve glycemic control and costs.
Evidence-Based Intervention Programs Across the Population Health Continuum
Diabetes Prevention (Preventive Care)
Prediabetes as a Significant Public Health Issue
Prediabetes is the high-risk state preceding type 2 diabetes and is typically identified by fasting glucose (100–125 mg/dl) or hemoglobin A1c (5.7–6.4%). Prediabetes can also be identified using a 75-g oral glucose tolerance test (2 h glucose, 140–199 mg/dl) [17]. Using these measures, approximately 38% of adults (86 million people) without diabetes are estimated to have prediabetes in the USA. Prediabetes is a significant marker of diabetes risk; 15 to 30% of people with prediabetes will develop type 2 diabetes in the next 5 years [18]. Most people with prediabetes (∼90%) in the USA are unaware of their prediabetes status [19].
Type 2 Diabetes Is Preventable Through Lifestyle Change at Population Level
Multiple randomized, controlled trials across the globe have demonstrated the efficacy of lifestyle modification for the prevention or delay of type 2 diabetes [20,21,22, 23••, 24,25,26,27,28,29] among those at high risk (Table 4). These trials published in the early 2000s conducted in Europe, India, East Asia, and the USA established that behavioral lifestyle interventions, through modest (5–7%) weight loss and increased physical activity, result in clinically significant reductions in diabetes risk over three to 6 years (relative risk reductions, 28.5 to 67.4%; absolute risk reductions, 6.3 to 21.7%; number needed to treat, 5 to 16). Long-term follow-up of participants in these randomized trials have demonstrated a persistent effect of a lifestyle intervention for reducing diabetes risk over ten or more years in the Diabetes Prevention Program Outcome Study [22, 23••]. Similarly, over 23 years of follow-up in the China Da Qing Diabetes Prevention Study (DPS), the risk of developing diabetes as well as all-cause and cardiovascular disease mortality was lower [29].
In 2008, Ackerman et al. published the results of the Diabetes Education and Prevention with a Lifestyle Intervention Offered at the YMCA (DEPLOY), in which the Diabetes Prevention Program (DPP) lifestyle intervention was adapted for a group setting. Compared to brief counseling alone (−1.8%), weight loss was 4.2 percentage points greater (P = 0.008) for the group-based DPP (−6.0%) at 12 months [30]. This landmark study demonstrated that the DPP could be implemented in a community setting at a low cost. In 2015, 16 years after the initial publication of the China Da Qing DPS [27], a comprehensive meta-analysis comparing lifestyle (diet + physical activity) to usual care found that lifestyle interventions [23••] in populations at risk lower weight by an average of 2.5% across settings; higher-intensity programs had larger effects [31].
Economic Evaluation of Lifestyle Interventions for Diabetes Prevention
Economic evaluations of lifestyle intervention for diabetes are promising from a cost effectiveness standpoint. In the DPP Outcomes Study, a within-trial analysis with a payer perspective and time horizon of 10 years demonstrated that lifestyle modification was cost-effective ($10,037 per quality-adjusted life year (QALY)) [32]. In a systematic review in 2015, Li et al., evaluated costs from 16 studies of diet and physical activity programs aimed at reducing diabetes risk and found that from a health system perspective, the median incremental cost-effectiveness ratio (ICER) was $13,761 per QALY with group-based program having much lower median costs ($1819/QALY) than individual-based ($15,846/QALY) programs [33].
Policy, Dissemination, and Implementation
The accumulating evidence on the effectiveness of lifestyle interventions targeting 5–7% weight loss and 150 min/week of moderate-intensity physical activity has sparked public health initiatives globally to reduce diabetes risk as reviewed in detail in a recent article [34]. For example, the Finnish National Diabetes Prevention Program (FIN-D2D) identified high-risk individuals using the FINDRISC (Finnish Diabetes Risk Score) for individual- and group-based lifestyle interventions; in this national program, 17.5% of participants lost at least 5% of their baseline weight at 1 year and were 69% less likely to develop diabetes during that time [35]. The Diabetes in Europe—Prevention Using Lifestyle, Physical Activity and Nutritional Intervention (DE-PLAN) was subsequently initiated to evaluate the impact of identifying (using FINDRISC) and intervening upon high-risk individuals across countries in Europe [36].
In the USA, under the Diabetes Prevention Act of 2009, the Centers for Disease Control (CDC) established the National Diabetes Prevention Program (NDPP), a national program intended to raise awareness of diabetes risk and to target those at high risk of diabetes for evidence-based lifestyle change interventions [37, 38]. The NDPP requires risk stratification for determining eligibility for a DPP based on the following: elevated weight for height, biochemical evidence of prediabetes (based on impaired fasting glucose, impaired glucose tolerance, or HbA1c) or history of gestational diabetes, and/or high scores on risk screeners that assess non-laboratory-based risk factors (e.g., age, family history) [39]. The NDPP has established criteria for programs to apply for CDC recognition based on their fidelity with the original DPP approach. Specifically, for a program to apply for CDC recognition status, it must use an approved curriculum that lasts for 12 months and be led by certified lifestyle coaches. These programs must start out with a more intensive phase (e.g., weekly in-person sessions) for the first 6 months followed by a maintenance period of 6 months. In order to attain full recognition, programs must meet specific attendance, physical activity, and weight loss goals (Table 5) at 6 and 12 months [39]. The CDC’s Diabetes Prevention Recognition Program (DPRP) requires regular reporting of data to the CDC to assess outcomes. As of January 8, 2017, of 1236 programs participating in the DPRP, 89 (7.2%) had attained full recognition.
The Medicare Diabetes Prevention Program
In March 2016, based on a demonstration among 6874 Medicare beneficiaries, the US Department of Health and Human Services announced its intention to cover the DPP as a benefit for Medicare members [40]. In this demonstration project, eligible Medicare members were recruited to YMCA DPPs: >80% attended at least four in-person group sessions, and of those attending at least four sessions, average weight loss was 4.7% for those attending ≥4 sessions and 5.2% for those attending ≥9 sessions over 24 months. Actuarial analyses demonstrated a cost savings of $2650 per enrollee over 15 months compared to members not in the program.
Diabetes Prevention Program Dissemination and Implementation in the USA
A major challenge to translating the evidence into clinical and public health practice is that the definitions of prediabetes, screening strategies, and treatment recommendations vary across several influential US organizations, including, the US Preventive Services Task Force (USPSTF), the American Diabetes Association (ADA), the American College of Endocrinology (ACE), the American Association of Clinical Endocrinologists (AACE), the American Association of Family Physicians, and the American College of Physicians. A similar challenge exists in assessing the effectiveness of inpatient glucose management programs, where professional societies and regulatory groups have not agreed upon uniform definitions of hypoglycemia, euglycemia, and hyperglycemia (see below, “Inpatient Diabetes Management (Acute Care)” section) The long-term benefit of pharmacologic therapy for diabetes prevention is also unknown. Finally, awareness and knowledge of prediabetes by all stakeholders is limited. Alignment around these issues and further study, particularly in the case of pharmacologic therapy, are needed.
Outpatient Diabetes Management and Patient Self-Management (Chronic Care)
Diabetes Self-Management Education and Support
Patient diabetes self-management education (DSME) is a standard of care, and research examining effectiveness of DSME has led to specific recommendations for the content and quality of DSME [41•, 42]. Several reviews have found evidence that group education, as compared to usual care, results in improvement in glycemic control, with mean changes in HbA1c ranging −0.4 to −1.4% at 6 months following education, −0.5 to −0.8% at 12 months, and −0.9 to −1.0% at 24 months [43, 44]. Studies show that group education also results in improvements in knowledge, self-management behaviors, self-efficacy, and patient satisfaction [44]. There is less evidence that individual education is more effective than usual care for clinical, behavioral, or psychosocial outcomes [43]. Patients with poorer HbA1c at baseline tend to have greater reductions in HbA1c following DSME [43, 45]. A meta-analysis of trials of culturally tailored educational interventions delivered to racial and ethnic minority patient subgroups with type 2 diabetes found a mean reduction in HbA1c of −0.4% at 3 months, −0.5% at 6 months, −0.2% at 12 months, and −0.3% at 24 months [46].
Provider and System Level Interventions to Improve Glycemic Control and Other Outcomes in the Ambulatory Setting [47]
Successful quality improvement strategies for ambulatory diabetes care target several areas—patients (patient education, promotion of self-management, reminder systems), healthcare providers (audit and feedback, clinician education, clinician reminders, financial incentives), and health systems (case management, team changes, electronic patient registry, facilitated relay of information to clinicians, continuous quality improvement [QI]) [48]. Several prior meta-analyses have examined the impact of these intervention approaches on glycemic control and other metabolic control indices in patients with diabetes [48,49,50].
Shojania et al. performed a systematic review and meta-analysis of 58 studies of 66 distinct trials incorporating these multiple intervention areas. The mean post-intervention HbA1c difference, compared to preintervention, was −0.42% with greater reductions if baseline HbA1c was ≥8% [49]. Strategies associated with at least a 0.5% reduction in HbA1c after controlling for baseline HbA1c ≥8% and study size included team changes (−0.67%) and case management (−0.52%). In comparative analyses, interventions that included case management reduced HbA1c significantly more than interventions that did not include case management and of these types of interventions, the most effective case management interventions were those in which the case managers could make independent medication changes [49]. This was confirmed in a subsequent meta-analysis of randomized controlled trials of disease management programs improving glycemic control in adults with type 1 and type 2 diabetes [50]. Similarly, interventions that included team changes reduced HbA1c significantly more than interventions that did not include team changes, particularly those that included multi-disciplinary, interactive teams [50]. Interventions with team changes remained significant after controlling for the presence of case management [49]. In those studies, adding a new team member alone was not effective but rather, adding a team member with shared care between specialists and primary care providers or new team members with an expanded role was most effective.
A more recent meta-analysis expanded on Shojania’s prior study by including process outcome measures and additional non-glycemic outcome measures to evaluate the additional impact of multi-component diabetes quality improvement interventions [48]. Overall, interventions resulted in lower HbA1c, LDL-cholesterol, and blood pressure in those receiving compared those not receiving the interventions [48]. These strategies also improved the likelihood that patients received aspirin therapy, anti-hypertensives, and screening for diabetic complications. Statin use, blood pressure control, and smoking cessation were unchanged. For patients with HbA1c ≥8% intervention strategies that lowered HbA1c ≥0.5% included team changes, case management, patient education, and promotion of self-management; however, for patients with HbA1c <8%, facilitated relay was more effective in lowering HbA1c ≥0.5% [48]. The only intervention strategy that was not effective in lowering HbA1c was clinician education alone [48]. These data suggest that greater improvements in HbA1c can be achieved utilizing multi-level QI intervention strategies that target the healthcare system and patient.
Ambulatory Interventions Targeting Underserved and Minority Populations [47]
Glazier et al. conducted a systematic review of 17 studies examining the effectiveness of patient, provider, and health system interventions among patients with type 1 or type 2 diabetes in socially disadvantages populations, defined as those of low socioeconomic status or belonging to an ethnic/racial minority group [51]. Eight of 13 studies showed improvements in HbA1c but less impact on body weight, lipids, and blood pressure. Features of effective interventions that lowered HbA1c included cultural and health literacy tailoring, leading by community educators or lay people, 1:1 (versus group) interventions with individualized assessment/reassessment, incorporation of treatment algorithms, focusing on behavior-related tasks, and providing feedback and high intensity interventions over a long duration [51].
Patient Interventions Within Healthcare Organizations
In Peek’s review of 17 studies of patient interventions within the healthcare organization that sought to improve dietary habits, physical activity, or self-management activities, those that were culturally tailored were more effective in lowering HbA1c than general QI interventions (−0.69 versus −0.1%) [52]. Also, peer support and 1:1 in-person diabetes self-management and patient education interventions were more effective than online and computer-based delivery modalities for self-management and patient education. In a systematic review and meta-analysis looking exclusively at randomized controlled trials of patient interventions targeting non-Hispanic Blacks (NHBs), most of which were culturally adapted and included peer providers, two of 22 increased patient attendance at screening visits for diabetic eye disease and 20 of 22 promoted improved diabetes self-management behaviors [53]. In a meta-analysis of eight studies, interventions resulted in a significant 0.83% reduction in HbA1c [53].
Community Health Worker Interventions
Systematic reviews of community health worker (CHW) interventions in diabetes published between 2006 and 2013 provide evidence of effectiveness of lay health worker interventions on outcomes including knowledge, diabetes self-care behaviors, clinical outcomes, and healthcare utilization and costs, largely for Hispanic and NHB populations [54,55,56]. Progress in delineating roles of CHWs (e.g., patient care, education, support for care delivery provided by other health professionals, care coordination, and social support) and training in scopes of practice for CHWs have been deemed elements of effective CHW use [54]. In addition to community-based provider service delivery, models of integration of CHW’s within healthcare teams [57, 58] have evidence of effectiveness in improving healthcare-related outcomes.
Provider Interventions
In Peek’s review, provider interventions including education, continuing medical education, computerized decision support, in-person feedback, and problem-based learning improved process measures [52]. The majority of these studies were conducted in NHBs with diabetes. Interventions involving computerized decision support reminders and chart audit and individual feedback resulted in improved HbA1c and treatment modification [59,60,61,62].
Healthcare Organization Interventions
Healthcare organization interventions in minority populations have included systems for rapid turnaround HbA1c, circumscribed appointments, support staff (e.g., nurse case management, community health worker, pharmacist), and increased follow-up through home visits or telephone/mail contact [52, 63]. In Peek’s review, 14 studies with interventions targeting the healthcare organization resulted in a mean HbA1c reduction of 0.34%. Ricci-Cabello et al. included five healthcare system intervention trials in NHBs in their systematic review and meta-analysis and found that the two most highly effective interventions in improving HbA1c and frequency of therapy intensification included rapid turnaround HbA1c [64].
Multi-target Interventions
Multi-target interventions target all aspects and components of healthcare delivery, including patients, providers, and the healthcare system. Five of these studies have targeted NHBs with diabetes and used various approaches. Three studies showed an improvement in HbA1c [65,66,67]. All of these interventions included patient education and self-management support and nurse case management, two included treatment algorithms [65, 66], and two involved collaboration with a physician in treatment decisions [65, 66]. While two additional multi-target interventions showed improvement in process measures and non-glycemic clinical outcomes [57, 68], they did not improve glycemic control. One study involved patient interventions and provider-focused QI interventions focused on system changes surrounding the physician visit [68] and the other involved nurse case management and community health workers using evidence-based clinical algorithms with feedback to primary care physicians [57]. Finally, one study focusing exclusively on Native Americans in the Indian Health Service included provider guidelines, a multi-disciplinary team, diabetes registry/tracking system, and flowsheets [69]. Compared to podiatric screening and patient education, the multi-target intervention resulted in a significant reduction in amputation rate [69].
Cost-Effectiveness of Ambulatory Interventions
Li and colleagues presented a systematic review on cost-effectiveness of diabetes interventions between 1985 and 2008 [70]. The following interventions were considered cost saving: ACE inhibitor therapy (ACEI) or angiotensin receptor blocker (ARB) for intensive hypertension control, ACEI or ARB treatment to prevent end-stage renal disease, and robust foot care to prevent ulcers. A number of other interventions, such as universal screening for diabetes in African-Americans who are 45–54 years old, intensive glycemic control in patients with newly diagnosed type 2 diabetes, intensive statin therapy, and others were found to be very cost-effective [70].
The 2005–2008 Medical Expenditure Panel Survey [71] found that patients with diabetes who received their care at a Community Health Center (CHC) saved payers and individuals up to $1656 in ambulatory care cost, compared to non-users of CHC. The quality of diabetes care was not different, compared to other primary care settings. Another study of four Midwestern CHC found that quality improvement of diabetes care may lead to additional administrative ($6–$22/patient, year 1) and clinical costs, though it varies between the centers [72].
Enhanced diabetes care may save money in the short run [73]. One study showed that improvement in HbA1c levels could potentially save between $685 and $950, mostly due to fewer hospital admissions, and reduced emergency room visits and physician consultations [74]. Ansell showed that improved access to primary care was associated with decreased utilization of non-urgent episodic care services among an indigent population in Chicago, Ill [75]. A 6-month diabetes group initiative at Kaiser Permanente resulted in reduced outpatient and hospital use [76].
In Europe, Nason et al. found that a multi-disciplinary foot protection clinic in Ireland resulted in €114,063 saving per year, as the number of major foot amputations decreased [77]. Schouten and colleagues found that enhanced patient-centered diabetes care in the Netherlands through quality improvement collaborations was modestly cost-effective [78]. While many interventions that intend to control diabetes may be cost-savings or at least cost-effective, a number of studies failed to show cost-effectiveness [79,80,81].
Inpatient Diabetes Management (Acute Care)
The number and percentage of hospitalized patients with diabetes has increased over the past two decades [82, 83], reflecting the increasing incidence and prevalence of diabetes [84, 85]. While individuals with diabetes represent 8–9% of the US population [86], they account for 23% of hospitalizations (approximately 8.8 million per year) [82]. Diabetes is also a significant source of healthcare expenditures. In 2007, of the projected $430 billion in national expenditures for inpatient hospital care, 23% ($97 billion) was incurred by individuals with diabetes [87]. In addition, admissions for uncontrollable diabetes, which are preventable, accounts for significant hospital costs as well, ranging from $552 million for those without complications to $1821 million for those with ketoacidosis [88]. In addition to having higher rates of hospital admission compared to non-diabetic individuals, those with diabetes also have longer lengths of stay [87]. There is also a cost associated with development of inpatient hypoglycemia. In one study, patients with diabetes who developed hypoglycemia during admission had significantly higher charges, longer length of stay, higher mortality, and greater odds of discharge to a skilled nursing facility [89]. Given the significant costs associated with acute care, there is a huge opportunity for cost savings by developing systems approaches to reducing length of stay and readmissions and preventing hospital admission for ambulatory sensitive conditions.
Because inpatient hypoglycemia and hyperglycemia are associated with negative clinical and economic outcomes, it is critical to devise systems approaches to improving the quality and safety of inpatient management of diabetes. In 2006, the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA) released a call to action outlining overarching strategies to successfully implement hospital-wide glucose control efforts to improve care of hospitalized patients with diabetes [90, 91]. The Joint Commission, in partnership with the ADA, bolstered this national movement by establishing key expectations for management of hospitalized patients with diabetes through its Advanced Certification in Inpatient Diabetes Program—(1) specific staff education requirements, (2) written blood glucose monitoring protocols, (3) plans for treatment of hypoglycemia and hyperglycemia, (4) data collection for indices of hypoglycemia, (5) patient education on diabetes self-management, and (6) identified program champion or champion team [92].
To achieve the recommended inpatient glycemic targets and outcomes (Tables 2 and 3), current recommendations include the presence of formal glucose management program infrastructure to facilitate development of standardized order sets, hypoglycemia tracking, and hypoglycemia protocols to deliver safe and high-quality care to hospitalized patients with diabetes [10, 12, 13, 91]. The most commonly employed quality improvement (QI) interventions in inpatient glucose management can be divided broadly into the following categories: (1) provider reminder systems and decision support [15, 93,94,95,96], (2) automated computer order entry [93, 97,98,99], (3) prescriber or nursing education [93, 95, 98, 100], and (4) organizational change [93, 96, 101, 102]. Most QI studies have used a multi-faceted approach as outlined by Draznin et al.’s conceptual model for systems interventions to improve quality and safety of inpatient glucose management (Fig. 2) [103].
A conceptual model for systems interventions to improve the quality and safety of inpatient management of hyperglycemia and diabetes. (Adapted with permission from: Draznin et al. Diabetes Care, 2013;36(7):1807–1814; permission conveyed through Copyright Clearance Center, Inc.) [103]
Interventions to Improve Glycemic Outcomes
Healthcare provider educational interventions
Provider education is an important component in improving diabetes processes of care and intermediary glucose outcomes [103]. Moreover, educating and utilizing existing staff (e.g., nurses, physicians) to implement safe glucose management is critical in settings that are not adequately staffed with endocrine subspecialists. Previous studies have shown that continuing nursing education can be effectively provided through the use of nurse educators, or “superusers”, who act as experts on institutional nursing policies and management principles, and are tasked with the peer-to-peer education of their unit-specific nurse colleagues [104, 105]. The advantages of the superuser model are that trained peers are (1) available to support their colleagues outside of traditional hours (since clinical activity occurs around the clock), (2) can be approached more comfortably with questions, and (3) can be available at the point of care [104]. The nursing superuser model has been applied successfully to hospital glycemic management. In one study describing a multi-component educational campaign for hypoglycemia prevention, nursing unit representatives developed expertise in the hypoglycemia protocol and communicated protocol changes back to their unit staff [106]. This intervention resulted in improved compliance with the hypoglycemia protocol and reduced hypoglycemic events [106]. Another program held “Train-the-Trainer” sessions for diabetes liaison nurses who became unit-based experts and clinical resources for the hospital’s insulin protocols [107]. This multi-component program resulted in a decline in median glucose for diabetic patients and percentage of patients experiencing hyperglycemia [107]. In our own hospital, we developed a diabetes nursing superuser program that was critical to implementing our hypoglycemia policy nursing interventions, contributing to a sustained reduction in hypoglycemia over 3 years in our Inpatient Glucose Management Program [108]. We are developing a similar diabetes superuser education program for our physicians in order to similarly impact hyperglycemia [109]. In three studies, incorporation of case-based education sessions into a hospital-wide glycemic improvement program resulted in decreased use of sliding scale insulin [98, 110], increased use of basal-bolus correction insulin [98, 110], greater modification of the glycemic regimen in response to severe hyperglycemia [111], and improved glycemic control [98, 110, 111], without an increase in hypoglycemia rates [98, 110, 111].
System-Level Interventions
In a large academic center, the combination of provider education and computerized insulin order sets resulted in a modest improvement in hyperglycemia without any significant increase in hypoglycemia [98]. In another example, between January 2006 and December 2009, a Glucose Steering Committee developed and implemented four hospital-wide programs to improve glucose management in patients with diabetes and hyperglycemia—(1) hospital-wide hypoglycemia policy and order set, (2) diabetes nursing superuser program, (3) hospital-wide hyperglycemia policy and order set, and (4) upgraded hyperglycemia order set with medical logic algorithms [112]. Among adult, non-intensive care unit (ICU) patients with diabetes and hyperglycemia, there was a 19% sustained reduction in hypoglycemic events over the course of these interventions [112].
Clinical Decision Support Systems
Various types of systems targeting glycemic control are now widely utilized in hospitals throughout the USA. These include computerized provider order entry (CPOE), computerized-based insulin dosing algorithms (CBIA), continuous glucose monitoring (CGM) with closed-loop insulin delivery, and glucose dashboards. A 2011 systematic review by Nirantharakumar summarized the evidence from 14 studies of these systems on glycemic outcomes among hospitalized patients with diabetes in the non-critical care setting. [113] With respect to CPOE-based interventions, most studies found improvements in rates of hyperglycemia with an overall average reduction in patient day-weighted mean blood glucose ranging from 10.8 to 15.6 mg/dl, with only one of the studies showing a significant increase in hypoglycemic events. [113] Since the publication of this meta-analysis, there have been several additional studies evaluating the impact of CPOEs on glycemic outcomes [112, 114, 115]. Most, but not all, of these studies showed similar reductions in hyperglycemia rates without worsening hypoglycemia. In the one recent null study [114], the lack of effect was attributed to low institutional uptake.
Several commercial CBIAs exist that provide automated titration of insulin infusions and subcutaneous insulin regimens in the hospital [116]. In a retrospective cross-over study, a nurse-directed eGlycemic Management System (eGMS) for subcutaneous basal-bolus insulin therapy achieved better glycemic control with less hypoglycemia than basal-bolus insulin therapy managed by providers. [117•] In recent years the use of CGM and closed loop insulin delivery have been considered as advanced methods of clinical decision support for hospitalized patients. A recent randomized parallel-group trial found that this technology improved glycemic control without increasing rates of hypoglycemia among inpatients with type 2 diabetes. [118] Despite the potential of closed loop technology, “widespread adoption of CGM by hospitals is limited by added costs and insufficient outcome data” [119].
Another common IT-based strategy for inpatient glycemic management is the use of glucose dashboards or reports to facilitate “active case finding of in-need patients” [113]. The majority of studies evaluating this intervention showed positive results, with reductions in both hypoglycemia and hyperglycemia rates [111, 120,121,122]. One study showed no significant improvements in glycemic control with the use of a “multi-component intervention that included an out-of-range glucose report derived electronically” [123]. The financial impact of glucose dashboards for health systems has not been formally studied. However, such tools clearly facilitate tracking of glucometric data at unit, hospital, and health system levels. The Society of Hospital Medicine sponsors a web-based data and reporting center that enables hospitals to compare performance in glycemic control against other hospitals and benchmarks [14]. If, in the future, the quality of inpatient glycemic control becomes a CMS metric tied to reimbursement, health systems will need to be able to readily furnish glucometric data through the use of standardized glucose dashboards.
Considering the complexity of inpatient glucose management, it is not surprising that both IT-based and non-IT-based strategies are often required in combination to achieve significant improvements in glycemic control. Some examples of successful non-IT based interventions include process changes related to timing of meal and insulin delivery [124], restriction of high-dose insulin ordering to endocrine consultants [125], and post-operative algorithms/care bundles [126, 127].
Interventions to Improve Hospital Costs and Length of Stay
Glucose Management Teams
Prior studies examining the use of specialized diabetes teams or endocrinologists to manage individual inpatients with diabetes resulted in better glycemic control and decreased length of stay compared to general internists’ management [128,129,130]. Diabetes educational policies targeting nurses, physician assistants, attendings, or patients have been associated with a decrease in length of stay [98, 131, 132].
System-Level Interventions
There are limited data about the impact of hospital-wide system-based glucose management programs on length of stay or hospital cost. One study examined the impact of an intensive glucose management protocol on economic outcomes in a mixed medical-surgical adult ICU. Compared to the preintervention period, there was a significant reduction in ICU and ventilator days; total lab, pharmacy, and radiology costs; post-ICU length of stay; and total hospital cost/patient [133]. In one study of a comprehensive inpatient diabetes management program that included diabetes education, a hypoglycemia policy, and computerized insulin order sets, there was a decrease in unadjusted length of stay in critical care and non-critical care patients [134]. In another study [135] of a multi-disciplinary diabetes care management program, there was a decrease in cost/admission. From a financial perspective, glycemic management in the post-operative period has been an area of focus for hospitals in light of the increased costs associated with surgical site infections associated with hyperglycemia. A systematic review and meta-analysis of 8515 patients found that surgical care bundles, of which glycemic control was one component, were associated with a 45% lower odds (95% CI 39–77%) of surgical site infection [127]. While there are presently insufficient economic data regarding the specific impact of glycemic control on surgical site infections [127], one study suggested a significant ROI with care bundles targeting this outcome, with estimated annual costs of $50,000 and savings of $234,261 (achieved mainly through reduction in LOS) [136].
Interventions to Improve Readmissions
Several interventions to reduce readmission risk among patients with diabetes have been explored in mostly small studies of variable quality [137]. One strategy that has been tested in randomized controlled trials (RCTs) is inpatient diabetes care by specialists, for which the data are mixed. One study found that daily rounds by a nurse diabetes educator and an endocrinologist decreased all-cause readmission rates within 3 months from 32 to 15% (P = 0.01) [129]. In contrast, however, another study reported that a diabetes specialty nurse decreased length of stay but did not affect readmission rate over 1 year [138]. There is some evidence supporting a beneficial effect of inpatient diabetes education (IDE) on readmission rates. A retrospective cohort study of 2265 hospitalized patients with uncontrolled diabetes reported that IDE was associated with a statistically significantly lower odds of readmission within 30 and 180 days [139]. A single-arm pilot of IDE and follow-up by phone after discharge in 82 patients with uncontrolled diabetes was associated with an 88.5% lower rate of hospitalization for severe hyperglycemia during 6 months of follow-up [140]. An RCT in 65 diabetes patients admitted for hypoglycemia found that IDE, medication adjustment, and discharge planning significantly reduced the risk of readmission for hypoglycemia while also reducing length of stay by more than 2 days [141]. In addition to inpatient diabetes care and education, data from a few retrospective studies suggest that intensifying diabetes therapy upon hospital discharge may reduce readmission risk among poorly controlled patients [142,143,144]. Another strategy for reducing readmission rates is outpatient diabetes specialty care. A pilot RCT found that follow-up at a diabetes transitional care clinic within 5 days of discharge significantly decreased the incidence of diabetes-related readmissions among patients admitted for diabetes [145]. A non-randomized study of diabetes specialty outpatient support appeared to decrease the risk of readmission for diabetic ketoacidosis over 2 years among patients with type 1 diabetes [146]. Although not formally tested, qualitative data suggest that readmission incidence may be reduced by improving the hospital discharge process with better communication of discharge instructions and involving patients more in discharge planning [147]. Another approach is to utilize CDSS, as was implemented in a health system of 13 hospitals as part of their CME Hospital Readmission Reduction Program and forthcoming bundled payment for patients admitted for coronary artery bypass surgery [148, 149]. They found that the use of an eGMS achieved lower rates of hyperglycemia and hypoglycemia as well as marked reductions in readmission for cardiovascular patients (coronary artery bypass graft, congestive heart failure, acute myocardial infarction) compared to standard care without the CBIA system [148, 150]. Lastly, another approach that has yet to be prospectively tested is to identify hospitalized patients at higher risk for readmission using a predictive model and then focus resources on those patients. One such model, the Diabetes Early Readmission Risk Indicator (DERRI), is to our knowledge the only validated tool developed specifically with diabetes patients [151••]. Whether readmission reduction strategies [137] will reduce overall healthcare costs remains unknown.
Conclusion
Diabetes population health management includes a continuum in patient care from diabetes prevention to chronic outpatient diabetes management to acute diabetes care in the hospital setting. Overall, there are limited data on the cost-effectiveness of multi-level ambulatory diabetes interventions or system-level glucose management intervention programs in the hospital setting, identifying these as important areas for future research. This will inform the best financial models for health systems to incorporate these strategies into diabetes population health programs. From a policy standpoint, professional societies and government organizations need to align around uniform definitions of, screening for, and treatment of prediabetes in order to fully translate research into public health practice. Finally, regulatory agencies should endorse metrics and expectations for hospital management of diabetes, as has been done for ambulatory diabetes management, to incentivize health systems to incorporate acute care into its diabetes population health programs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021–9. This article contains the most up-to-date diabetes prevalence estimates for the United States.
Courtney-Long EA, Carroll DD, Zhang QC, Stevens AC, Griffin-Blake S, Armour BS, et al. Prevalence of disability and disability type among adults—United States, 2013. Mmwr-Morbid Mortal W. 2015;64(29):777–83.
Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. National Center for Health Statistics, Division of Vital Statistics. 2016;65(4):1–122.
Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, et al. US spending on personal health care and public health, 1996-2013. JAMA. 2016;316(24):2627–46.
Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–639.
National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities, Hyattsville, MD, 2016.
Kindig DA. Understanding population health terminology. Milbank Q. 2007;85(1):139–61.
Stiefel M, Nolan K. A guide to measuring the triple aim: population health, experience of care, and per capital costs. Cambridge: Institute for Healthcare Improvement; 2012.
Dunn JR, Hayes MV. Toward a lexicon of population health. Canadian Journal of Public Health-Revue Canadienne De Sante Publique. 1999;90:S7–S10.
Mathioudakis N, Golden SH. A comparison of inpatient glucose management guidelines: implications for patient safety and quality. Current Diabetes Reports. 2015;15(3):13.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–69.
American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–80.
Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
Maynard G, Schnipper JL, Messler J, Ramos P, Kulasa K, Nolan A, et al. Design and implementation of a web-based reporting and benchmarking center for inpatient glucometrics. Journal of diabetes science and technology. 2014;8(4):630–40.
Thomas P, Inzucchi SE. An internet service supporting quality assessment of inpatient glycemic control. Journal of diabetes science and technology. 2008;2(3):402–8.
https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index.html?redirect=/qualitymeasures/. Accessed 9 Mar 2017.
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–24.
National Center for Chronic Disease Prevention and Health Promotion Division of Diabetes Translation: about prediabetes and type 2 diabetes. 2016. http://www.cdc.gov/diabetes/prevention/prediabetes-type2/index.html.
Centers for Disease Control and Prevention. Awareness of prediabetes—United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209–12.
Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67(2):152–62.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEngl J Med. 2002;346(6):393–403.
Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi:10.1016/S0140-6736(09)61457-4.
•• Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. The lancet Diabetes & endocrinology. 2015;3(11):866–75. This article summarizes the long-term effects of the Diabetes Prevention Program interventions on the risk of microvascular complications of diabetes.
Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, et al. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243–51.
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50.
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97.
Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44.
Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–9.
Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. The lancet Diabetes & endocrinology. 2014;2(6):474–80.
Ackermann RT, Finch EA, Caffrey HM, Lipscomb ER, Hays LM, Saha C. Long-term effects of a community-based lifestyle intervention to prevent type 2 diabetes: the DEPLOY extension pilot study. Chronic illness. 2011;7(4):279–90.
Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):437–51.
Diabetes Prevention Program Research G. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35(4):723–30.
Li R, Qu S, Zhang P, Chattopadhyay S, Gregg EW, Albright A, et al. Economic evaluation of combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):452–60.
Cefalu WT, Buse JB, Tuomilehto J, Fleming GA, Ferrannini E, Gerstein HC, et al. Update and next steps for real-world translation of interventions for type 2 diabetes prevention: reflections from a diabetes care editors’ expert forum. Diabetes Care. 2016;39(7):1186–201.
Saaristo T, Moilanen L, Korpi-Hyovalti E, Vanhala M, Saltevo J, Niskanen L, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care. 2010;33(10):2146–51.
Schwarz PE, Lindstrom J, Kissimova-Scarbeck K, Szybinski Z, Barengo NC, Peltonen M, et al. The European perspective of type 2 diabetes prevention: diabetes in Europe—prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2008;116(3):167–72.
National Diabetes Prevention Program. https://www.congress.gov/bill/111th-congress/house-bill/4124. Accessed 8 Mar 2017.
Diabetes Prevention Recognition Program. https://nccd.cdc.gov/DDT_DPRP/Registry.aspx Accessed 8 Jan 2017.
Center for Disease Control and Prevention diabetes prevention recognition program: Standards and operating procedures. 2015. http://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf. Accessed 8 Mar 2017.
Office of the Actuary. Certification of Medicare diabetes prevention program. Baltimore: Centers for Medicare and Medicaid Services; 2016.
• Powers MA, Bardsley J, Cypress M, Duker P, Funnell MM, Hess Fischl A, et al. Diabetes self-management education and support in type 2 diabetes: a Joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care. 2015;38(7):1372–82. This article provides current state of the art recommendations for diabetes self-management education and support for patients with type 2 diabetes.
American Diabetes Association. Lifestyle management. Diabetes Care. 2017;40(Suppl. 1):S33–43.
Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. The Cochrane database of systematic reviews. 2009;1:CD005268.
Steinsbekk A, Rygg LO, Lisulo M, Rise MB, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
Sigurdardottir AK, Jonsdottir H, Benediktsson R. Outcomes of educational interventions in type 2 diabetes: WEKA data-mining analysis. Patient Educ Couns. 2007;67(1–2):21–31.
Attridge M, Creamer J, Ramsden M, Cannings-John R, Hawthorne K. Culturally appropriate health education for people in ethnic minority groups with type 2 diabetes mellitus. The Cochrane database of systematic reviews. 2014;9:CD006424.
Joseph JJ, Golden SH. Diabetes in native populations and underserved communities in the USA. In: Dagogo-Jack S, editor. Diabetes mellitus in developing and underserved communities. Switzerland: Springer International Publishing; 2017. p. 251–84.
Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252–61.
Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296(4):427–40.
Pimouguet C, Le Goff M, Thiebaut R, Dartigues JF, Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2011;183(2):E115–27.
Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care. 2006;29(7):1675–88.
Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64(5):101S–56S.
Ricci-Cabello I, Ruiz-Perez I, Olry dL, Marquez-Calderon S. Do social inequalities exist in terms of the prevention, diagnosis, treatment, control and monitoring of diabetes? A systematic review. Health Soc Care Community. 2010;18(6):572–87.
Shah M, Kaselitz E, Heisler M. The role of community health workers in diabetes: update on current literature. Current diabetes reports. 2013;13(2):163–71.
Hunt CW, Grant JS, Appel SJ. An integrative review of community health advisors in type 2 diabetes. J Community Health. 2011;36(5):883–93.
Norris SL, Chowdhury FM, Van Le K, Horsley T, Brownstein JN, Zhang X, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2006;23(5):544–56.
Gary TL, Batts-Turner M, Yeh HC, Hill-Briggs F, Bone LR, Wang NY, et al. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2009;169(19):1788–94.
Kane EP, Collinsworth AW, Schmidt KL, Brown RM, Snead CA, Barnes SA, et al. Improving diabetes care and outcomes with community health workers. Fam Pract. 2016;33(5):523–8.
Fox CH, Mahoney MC. Improving diabetes preventive care in a family practice residency program: a case study in continuous quality improvement. Fam Med. 1998;30(6):441–5.
Phillips LS, Hertzberg VS, Cook CB, El-Kebbi IM, Gallina DL, Ziemer DC, et al. The Improving Primary Care of African Americans with Diabetes (IPCAAD) project: rationale and design. Control Clin Trials. 2002;23(5):554–69.
Phillips LS, Ziemer DC, Doyle JP, Barnes CS, Kolm P, Branch WT, et al. An endocrinologist-supported intervention aimed at providers improves diabetes management in a primary care site: improving primary care of African Americans with diabetes (IPCAAD) 7. Diabetes Care. 2005;28(10):2352–60.
Ziemer DC, Tsui C, Caudle J, Barnes CS, Dames F, Phillips LS. An informatics-supported intervention improves diabetes control in a primary care setting. AMIA annual symposium proceedings/amia symposium amia symposium. 2006:1160.
Ricci-Cabello I, Ruiz-Perez I, Nevot-Cordero A, Rodriguez-Barranco M, Sordo L, Goncalves DC. Health care interventions to improve the quality of diabetes care in African Americans: a systematic review and meta-analysis. Diabetes Care. 2013;36(3):760–8.
Thaler LM, Ziemer DC, Gallina DL, Cook CB, Dunbar VG, Phillips LS, et al. Diabetes in urban African-Americans. XVII. Availability of rapid HbA1c measurements enhances clinical decision-making. Diabetes Care. 1999;22(9):1415–21.
Cook CB, Penman A, Cobb AB, Miller D, Murphy T, Horn T. Outpatient diabetes management of Medicare beneficiaries in four Mississippi fee-for-service primary care clinics. J Miss State Med Assoc. 1999;40(1):8–13.
Bray P, Thompson D, Wynn JD, Cummings DM, Whetstone L. Confronting disparities in diabetes care: the clinical effectiveness of redesigning care management for minority patients in rural primary care practices. The Journal of rural health: official journal of the American Rural Health Association and the National Rural Health Care Association. 2005;21(4):317–21.
Anderson-Loftin W, Barnett S, Sullivan P, Bunn PS, Tavakoli A. Culturally competent dietary education for southern rural African Americans with diabetes. The Diabetes educator. 2002;28(2):245–57.
Mahotiere T, Ocepek-Welikson K, Daley MB, Byssainthe JP. A program to reduce the disparity in the rate of biennial lipid profiles between African-American and white Medicare beneficiaries with diabetes mellitus in New York City. J Community Health. 2006;31(4):263–88.
Rith-Najarian S, Branchaud C, Beaulieu O, Gohdes D, Simonson G, Mazze R. Reducing lower-extremity amputations due to diabetes—application of the staged diabetes management approach in a primary care setting. J Fam Pract. 1998;47(2):127–32.
Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33(8):1872–94.
Richard P, Shin P, Beeson T, Burke LS, Wood SF, Rosenbaum S. Quality and cost of diabetes mellitus care in Community Health Centers in the United States. PLoS One. 2015;10(12):e0144075.
Huang ES, Brown SE, Zhang JX, Kirchhoff AC, Schaefer CT, Casalino LP, et al. The cost consequences of improving diabetes care: the community health center experience. Joint Commission journal on quality and patient safety/Joint Commission Resources. 2008;34(3):138–46.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–9.
Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182–9.
Ansell D, Schiff R, Goldberg D, Furumoto-Dawson A, Dick S, Peterson C. Primary care access decreases nonurgent hospital visits for indigent diabetics. J Health Care Poor Underserved. 2002;13(2):171–83.
Sadur CN, Moline N, Costa M, Michalik D, Mendlowitz D, Roller S, et al. Diabetes management in a health maintenance organization. Efficacy of care management using cluster visits. Diabetes Care. 1999;22(12):2011–7.
Nason GJ, Strapp H, Kiernan C, Moore K, Gibney J, Feeley TM, et al. The cost utility of a multi-disciplinary foot protection clinic (MDFPC) in an Irish hospital setting. Ir J Med Sci. 2013;182(1):41–5.
Schouten LM, Niessen LW, van de Pas JW, Grol RP, Hulscher ME. Cost-effectiveness of a quality improvement collaborative focusing on patients with diabetes. Med Care. 2010;48(10):884–91.
Wagner EH, Grothaus LC, Sandhu N, Galvin MS, McGregor M, Artz K, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24(4):695–700.
de Weerdt I, Visser AP, Kok GJ, de Weerdt O, van der Veen EA. Randomized controlled multicentre evaluation of an education programme for insulin-treated diabetic patients: effects on metabolic control, quality of life, and costs of therapy. Diabet Med. 1991;8(4):338–45.
Dougherty G, Schiffrin A, White D, Soderstrom L, Sufrategui M. Home-based management can achieve intensification cost-effectively in type I diabetes. Pediatrics. 1999;103(1):122–8.
HCUP Nationwide Inpatient Sample (NIS). Agency for healthcare research and quality. 2010. http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed 5 June 2014:2014.
CDC. Number (in thousands) of hospital discharges with diabetes as any-listed diagnosis, United States, 1988–2009. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. http://www.cdc.gov/diabetes/statistics/dmany/fig1.htm. Accessed 11 June 2014:2014.
Centers for Disease C, Prevention. Increasing prevalence of diagnosed diabetes—United States and Puerto Rico, 1995-2010. MMWR Morb Mortal Wkly Rep. 2012;61(45):918–21.
Jones AP, Homer JB, Murphy DL, Essien JD, Milstein B, Seville DA. Understanding diabetes population dynamics through simulation modeling and experimentation. Am J Public Health. 2006;96(3):488–94.
Centers for Disease C, Prevention. National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011.2012. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
American Diabetes Association. Economic costs of diabetes in the U.S. Diabetes Care. 2008;31(3):1–20.
Kim S. Burden of hospitalizations primarily due to uncontrolled diabetes: implications of inadequate primary health care in the United States. Diabetes Care. 2007;30(5):1281–2.
Curkendall SM, Natoli JL, Alexander CM, Nathanson BH, Haidar T, Dubois RW. Economic and clinical impact of inpatient diabetic hypoglycemia. Endocr Pract. 2009;15(4):302–12.
ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Endocr Pract. 2006;12(Suppl 3):4–13.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31.
The Joint Commission. Advanced certification in inpatient diabetes. The Joint Commission. http://www.jointcommission.org/certification/inpatient_diabetes.aspx. Accessed 8 Mar 2017.
Munoz C, Lowry C, Smith C. Continuous quality improvement: hypoglycemia prevention in the postoperative surgical population. Medsurg Nurs. 2012;21(5):275–80.
Trujillo JM, Barsky EE, Greenwood BC, Wahlstrom SA, Shaykevich S, Pendergrass ML, et al. Improving glycemic control in medical inpatients: a pilot study. J Hosp Med. 2008;3(1):55–63.
Herring R, Pengilley C, Hopkins H, Tuthill B, Patel N, Nelson C, et al. Can an interprofessional education tool improve healthcare professional confidence, knowledge and quality of inpatient diabetes care: a pilot study? Diabet Med. 2013;30(7):864–70.
Donaldson S, Villanuueva G, Rondinelli L, Baldwin D. Rush University guidelines and protocols for the management of hyperglycemia in hospitalized patients: elimination of the sliding scale and improvement of glycemic control throughout the hospital. The Diabetes educator. 2006;32(6):954–62.
Lee J, Clay B, Zelazny Z, Maynard G. Indication-based ordering: a new paradigm for glycemic control in hospitalized inpatients. Journal of diabetes science and technology. 2008;2(3):349–56.
Schnipper JL, Ndumele CD, Liang CL, Pendergrass ML. Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27.
Flanders SJ, Juneja R, Roudebush CP, Carroll J, Golas A, Elias BL. Glycemic control and insulin safety: the impact of computerized intravenous insulin dosing. American journal of medical quality: the official journal of the American College of Medical Quality. 2009;24(6):489–97.
Gilman JA. A quality improvement project for better glycemic control in hospitalized patients with diabetes. The Diabetes educator. 2001;27(4):541–6.
Pasala S, Dendy JA, Chockalingam V, Meadows RY. An inpatient hypoglycemia committee: development, successful implementation, and impact on patient safety. Ochsner J. 2013;13(3):407–12.
Korytkowski M, Dinardo M, Donihi AC, Bigi L, Devita M. Evolution of a diabetes inpatient safety committee. Endocr Pract. 2006;12(Suppl 3):91–9.
Draznin B, Gilden J, Golden SH, Inzucchi SE, PRIDE investigators, Baldwin D, et al. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care. 2013;36(7):1807–14.
Boffa DP, Pawola LM. Identification and conceptualization of nurse super users. J Healthc Inf Manag. 2006;20(4):60–8.
McIntire S, Clark T. Essential steps in super user education for ambulatory clinic nurses. Urol Nurs. 2009;29(5):337–42. quiz 43
Selig PM, Popek V, Peebles KM. Minimizing hypoglycemia in the wake of a tight glycemic control protocol in hospitalized patients. J Nurs Care Qual. 2010;25(3):255–60.
Murphy DM, Vercruysse RA, Bertucci TM, Wall MJ, Schriever AE, Nabhan FA, et al. Reducing hyperglycemia hospitalwide: the basal-bolus concept. Joint Commission journal on quality and patient safety/Joint Commission Resources. 2009;35(4):216–23.
Kemmerer T, Bashura H, Dintzis J, Mathioudakis N, Golden SH. The impact of nursing and advanced practice clinician on the implementation and outcomes of an inpatient glucose management program. AADE In Practice. 2015:17–25.
Golden SH, Hager D, Gould LJ, Mathioudakis N, Pronovost P. A gap analysis needs assessment tool to drive a care delivery and research agenda for integration of care and sharing of best practices across a health system. The Joint Commission Journal on Quality and Patient Safety. 2017;43:18–28.
Ena J, Casan R, Lozano T, Leach A, Algado JT, Navarro-Diaz FJ. Long-term improvements in insulin prescribing habits and glycaemic control in medical inpatients associated with the introduction of a standardized educational approach. Diabetes Res Clin Pract. 2009;85(2):159–65.
Donihi AC, Gibson JM, Noschese ML, DiNardo MM, Koerbel GL, Curll M, et al. Effect of a targeted glycemic management program on provider response to inpatient hyperglycemia. Endocr Pract. 2011;17(4):552–7.
Munoz M, Pronovost P, Dintzis J, Kemmerer T, Wang NY, Chang YT, et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Joint Commission journal on quality and patient safety/Joint Commission Resources. 2012;38(5):195–206.
Nirantharakumar K, Chen YF, Marshall T, Webber J, Coleman JJ. Clinical decision support systems in the care of inpatients with diabetes in non-critical care setting: systematic review. Diabet Med. 2012;29(6):698–708.
Wong B, Mamdani MM, Yu CH. Computerized insulin order sets and glycemic control in hospitalized patients. Am J Med. 2016; doi:10.1016/j.amjmed.2016.09.034.
Neubauer KM, Mader JK, Höll B, Aberer F, Donsa K, Augustin T, et al. Standardized glycemic management with a computerized workflow and decision support system for hospitalized patients with type 2 diabetes on different wards. Diabetes Technol Ther. 2015;17(10):685–92.
Gianchandani R, Umpierrez GE. Inpatient use of computer-guided insulin devices moving into the non-intensive care unit setting. Diabetes Technol Ther. 2015;17(10):673–5.
• Aloi J, Bode BW, Ullal J, Chidester P, McFarland RS, Bedingfield AE, et al. Comparison of an electronic glycemic management system versus provider-managed subcutaneous basal bolus insulin therapy in the hospital setting. J Diabetes Sci Technol. 2016; doi:10.1177/1932296816664746. This study showed that Electronic Glycemic Management System (eGMS) achieved glycemic control with less hypoglycemia compared to basal-bolus insulin managed by a prescriber.
Thabit H, Hartnell S, Allen JM, Lake A, Wilinska ME, Ruan Y, et al. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 2016; doi:10.1016/S2213-8587(16)30280-7.
Wallia A, Umpierrez GE, Nasraway SA, Klonoff DC, Investigators P. Round table discussion on inpatient use of continuous glucose monitoring at the International Hospital Diabetes Meeting. J Diabetes Sci Technol. 2016;10(5):1174–81.
Mendez CE, Ata A, Rourke JM, Stain SC, Umpierrez G. Daily inpatient glycemic survey (DINGS): a process to remotely identify and assist in the management of hospitalized patients with diabetes and hyperglycemia. Endocr Pract. 2015;21(8):927–35.
Boaz M, Landau Z, Matas Z, Wainstein J. Institutional blood glucose monitoring system for hospitalized patients: an integral component of the inpatient glucose control program. J Diabetes Sci Technol. 2009;3(5):1168–74.
O’Neill AE, Miranda D. The right tools can help critical care nurses save more lives. Crit Care Nurs Q. 2006;29(4):275–81.
Thompson R, Schreuder AB, Wisse B, Jarman K, Givan K, Suhr L, et al. Improving insulin ordering safely: the development of an inpatient glycemic control program. J Hosp Med. 2009;4(7):E30–5.
Engle M, Ferguson A, Fields W. A journey to improved inpatient glycemic control by redesigning meal delivery and insulin administration. Clin Nurse Spec. 2016;30(2):117–24.
Lee SY, Askin G, McDonnell ME, Arnold LM, Alexanian SM. Hypoglycemia rates after restriction of high-dose glargine in hospitalized patients. Endocr Pract. 2016; doi:10.4158/EP161288.OR.
Arnold LM, Mahesri M, McDonnell ME, Alexanian SM. Glycemic outcomes three years after implementation of a perioperative glycemic control algorithm in an academic institution. Endocr Pract. 2016; doi:10.4158/EP161354.OR.
Tanner J, Padley W, Assadian O, Leaper D, Kiernan M, Edmiston C. Do surgical care bundles reduce the risk of surgical site infections in patients undergoing colorectal surgery? A systematic review and cohort meta-analysis of 8,515 patients. Surgery. 2015;158(1):66–77.
Levetan CS, Salas JR, Wilets IF, Zumoff B. Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes. Am J Med. 1995;99(1):22–8.
Koproski J, Pretto Z, Poretsky L. Effects of an intervention by a diabetes team in hospitalized patients with diabetes. Diabetes Care. 1997;20(10):1553–5.
Simmons D, Hartnell S, Watts J, Ward C, Davenport K, Gunn E, et al. Effectiveness of a multidisciplinary team approach to the prevention of readmission for acute glycaemic events. Diabetic medicine: a journal of the British Diabetic Association. 2015;32(10):1361–7.
Feddersen E, Lockwood DH. An inpatient diabetes educator’s impact on length of hospital stay. The Diabetes educator. 1994;20(2):125–8.
Horton WB, Weeks AQ, Rhinewalt JM, Ballard RD, Asher FH. Analysis of a guideline-derived resident educational program on inpatient glycemic control. South Med J. 2015;108(10):596–8.
Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients. Chest. 2006;129(3):644–50.
Newton CA, Young S. Financial implications of glycemic control: results of an inpatient diabetes management program. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2006;12(Suppl 3):43–8.
Rasekaba TM, Lim WK, Hutchinson AF. Effect of a chronic disease management service for patients with diabetes on hospitalisation and acute care costs. Australian health review: a publication of the Australian Hospital Association. 2012;36(2):205–12.
Crolla RM, van der Laan L, Veen EJ, Hendriks Y, van Schendel C, Kluytmans J. Reduction of surgical site infections after implementation of a bundle of care. PLoS One. 2012;7(9):e44599.
Rubin DJ. Hospital readmission of patients with diabetes. Curr Diab Rep. 2015;15(4):1–9.
Davies M, Dixon S, Currie CJ, Davis RE, Peters JR. Evaluation of a hospital diabetes specialist nursing service: a randomized controlled trial. Diabet Med. 2001;18(4):301–7.
Healy SJ, Black D, Harris C, Lorenz A, Dungan KM. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care. 2013;36(10):2960–7.
Dungan K, Lyons S, Manu K, Kulkarni M, Ebrahim K, Grantier C, et al. An individualized inpatient diabetes education and hospital transition program for poorly controlled hospitalized patients with diabetes. Endocr Pract. 2014:1–24.
Kampan P. Effects of counseling and implementation of clinical pathway on diabetic patients hospitalized with hypoglycemia. J Med Assoc Thail. 2006;89(5):619–25.
Wei NJ, Wexler DJ, Nathan DM, Grant RW. Intensification of diabetes medication and risk for 30-day readmission. Diabet Med. 2013;30(2):e56–62.
Wu EQ, Zhou S, Yu A, Lu M, Sharma H, Gill J, et al. Outcomes associated with post-discharge insulin continuity in US patients with type 2 diabetes mellitus initiating insulin in the hospital. Hosp Pract (1995). 2012;40(4):40–8.
Lee PH, Franks AS, Barlow PB, Farland MZ. Hospital readmission and emergency department use based on prescribing patterns in patients with severely uncontrolled type 2 diabetes mellitus. Diabetes Technol Ther. 2014;16(3):150–5.
Seggelke SA, Hawkins RM, Gibbs J, Rasouli N, Wang C, Draznin B. Transitional care clinic for uninsured and Medicaid-covered patients with diabetes mellitus discharged from the hospital: a pilot quality improvement study. Hosp Pract (1995). 2014;42(1):46–51.
Maldonado MR, D’Amico S, Rodriguez L, Iyer D, Balasubramanyam A. Improved outcomes in indigent patients with ketosis-prone diabetes: effect of a dedicated diabetes treatment unit. Endocr Pract. 2003;9(1):26–32.
Rubin D, Donnell-Jackson K, Jhingan R, Golden SH, Paranjape A. Early readmission among patients with diabetes: a qualitative assessment of contributing factors. J Diabetes Complicat. 2014;28(6):869–73.
Dramatic readmission reductions for CABG, CHF, and AMI attributed to Glytec’s eGlycemic Management System. 2016. https://www.glytecsystems.com/News/dramatic-readmission-reductions-for-cabg-chf-and-ami-attributed-to-glytec-s-eglycemic-management-system.html. Accessed 28 Nov 2016.
Centers for Medicare & Medicaid services: readmissions reduction program. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. November 28, 2016.
Diabetes Technology Meeting. 2016. https://www.diabetestechnology.org/dtm/submitted-abstracts.shtml. Accessed 28 Nov 2016.
•• Rubin DJ, Handorf EA, Golden SH, Nelson DB, McDonnell ME, Zhao H. Development and validation of a novel tool to predict hospital readmission risk among patients with diabetes. Endocr Pract. 2016;22(10):1204–15. This article presents a novel risk prediction tool to identify patients at risk for 30-day readmissions based on readily available clinical and sociodemographic data.
Acknowledgments
Nisa Maruthur reports grants from the Baltimore City Health Department and from the Maryland Department of Health and Mental Hygiene. Daniel Rubin reports grants from NIH/NIDDK (K23DK102963). Nestoras Mathioudakis reports grant from NIH/NIDDK (K23DK111986-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sherita Hill Golden, Nisa Maruthur, Nestoras Mathioudakis, Elias Spanakis, and Mihail Zilbermint declare that they have no conflict of interest.
Daniel Rubin reports grants from Merck, Boehringer Ingelheim, and AstraZeneca.
Felicia Hill-Briggs is a member of the Board of Directors of the American Diabetes Association.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hospital Management of Diabetes
Rights and permissions
About this article
Cite this article
Golden, S.H., Maruthur, N., Mathioudakis, N. et al. The Case for Diabetes Population Health Improvement: Evidence-Based Programming for Population Outcomes in Diabetes. Curr Diab Rep 17, 51 (2017). https://doi.org/10.1007/s11892-017-0875-2
Published:
DOI: https://doi.org/10.1007/s11892-017-0875-2