Abstract
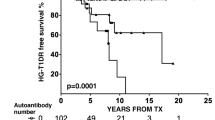
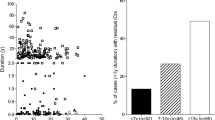
Type 1 diabetes recurrence (T1DR) affecting pancreas transplants was first reported in recipients of living-related pancreas grafts from twins or HLA identical siblings; given HLA identity, recipients received no or minimal immunosuppression. This observation provided critical evidence that type 1 diabetes (T1D) is an autoimmune disease. However, T1DR is traditionally considered very rare in immunosuppressed recipients of pancreas grafts from organ donors, representing the majority of recipients, and immunological graft failures are ascribed to chronic rejection. We have been performing simultaneous pancreas–kidney (SPK) transplants for over 25 years and find that 6–8 % of our recipients develop T1DR, with symptoms usually becoming manifest on extended follow-up. T1DR is typically characterized by (1) variable degree of insulitis and loss of insulin staining, on pancreas transplant biopsy (with most often absent), minimal to moderate and rarely severe pancreas, and/or kidney transplant rejection; (2) the conversion of T1D-associated autoantibodies (to the autoantigens GAD65, IA-2, and ZnT8), preceding hyperglycemia by a variable length of time; and (3) the presence of autoreactive T cells in the peripheral blood, pancreas transplant, and/or peripancreatic transplant lymph nodes. There is no therapeutic regimen that so far has controlled the progression of islet autoimmunity, even when additional immunosuppression was added to the ongoing chronic regimens; we hope that further studies and, in particular, in-depth analysis of pancreas transplant biopsies with recurrent diabetes will help identify more effective therapeutic approaches.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Burke GW, Ciancio G, Sollinger HW. Advances in pancreas transplantation. Transplantation. 2004;77(9 Suppl):S62–S7.
Ciancio G, Burke GW, Miller J. Current treatment practices in immunosuppression. Expert Opin Pharmacother. 2000;1:1307–30.
Ciancio G, Mattiazzi A, Miller J, Burke GW. Daclizumab as induction therapy in kidney and simultaneous pancreas-kidney transplantation. Minerva Urol Nefrol. 2003;55(1):43–56.
Burke GW, Ciancio G. Critical care issues in the renal and pancreatic allograft recipient. In: Civetta JM, Taylor RW, Kirby RR, editors. Critical care. 3rd ed. Philadelphia: J.B. Lippincott Company; 1997. p. 1311–5.
Burke III GW, Ciancio G, Figueiro J, Buigas R, Olson L, Roth D, et al. Hypercoagulable state associated with kidney-pancreas transplantation. Thromboelastogram-directed anti-coagulation and implications for future therapy. Clin Transplant. 2004;18(4):423–8.
Burke GW, Ciancio G, Cirocco R, Markou M, Olson L, Contreras N, et al. Microangiopathy in kidney and simultaneous pancreas/kidney recipients treated with tacrolimus: evidence of endothelin and cytokine involvement. Transplantation. 1999;68(9):1336–42.
Moon JI, Ciancio G, Burke GW. Arterial reconstruction with donor iliac vessels during pancreas transplantation: an intraoperative approach to arterial injury or inadequate flow. Clin Transplant. 2005;19(2):286–90.
Burke III GW, Kaufman DB, Millis JM, Gaber AO, Johnson CP, Sutherland DE, et al. Prospective, randomized trial of the effect of antibody induction in simultaneous pancreas and kidney transplantation: three-year results. Transplantation. 2004;77(8):1269–75.
Ciancio G, Sageshima J, Chen L, Gaynor JJ, Hanson L, Tueros L, et al. Advantage of rapamycin over mycophenolate mofetil when used with tacrolimus for simultaneous pancreas kidney transplants: randomized, single-center trial at 10 years. Am J Transplant. 2012;12(12):3363–76.
Burke GW, Ciancio G, Olson L, Roth D, Miller J. Ten-year survival after simultaneous pancreas/kidney transplantation with bladder drainage and tacrolimus-based immunosuppression. Transplant Proc. 2001;33(1–2):1681–3.
Sutherland DE, Sibley R, Xu XZ, Michael A, Srikanta AM, Taub F, et al. Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of type I diabetes. Trans Assoc Am Physicians. 1984;97:80–7.
Sibley RK, Sutherland DE, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest; J Tech Methods Pathol. 1985;53(2):132–44.
Sutherland DE, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes. 1989;38(Supplement 1):85–7.
Nakhleh RE, Gruessner RW, Swanson PE, Tzardis PJ, Brayman K, Dunn DL, et al. Pancreas transplant pathology. A morphologic, immunohistochemical, and electron microscopic comparison of allogeneic grafts with rejection, syngeneic grafts, and chronic pancreatitis. Am J Surg Pathol. 1991;15(3):246–56.
Santamaria P, Nakhleh RE, Sutherland DE, Barbosa JJ. Characterization of T lymphocytes infiltrating human pancreas allograft affected by isletitis and recurrent diabetes. Diabetes. 1992;41(1):53–61.
Bosi E, Bottazzo GF, Secchi A, Pozza G, Shattock M, Saunders A, et al. Islet cell autoimmunity in type I diabetic patients after HLA-mismatched pancreas transplantation. Diabetes. 1989;38 Suppl 1:82–4.
Dieterle CD, Hierl FX, Gutt B, Arbogast H, Meier GR, Veitenhansl M, et al. Insulin and islet autoantibodies after pancreas transplantation. Transpl Int. 2005;18(12):1361–5.
Lohmann T, Klemm T, Geissler F, Uhlmann D, Ludwig S, Hauss J, et al. Islet cell-specific autoantibodies as potential markers for recurrence of autoimmune type 1 diabetes after simultaneous pancreas-kidney transplantation. Transplant Proc. 2002;34(6):2249–50.
Sundkvist G, Tyden G, Karlsson FA, Bolinder J. Islet autoimmunity before and after pancreas transplantation in patients with type I diabetes mellitus [letter] [in process citation]. Diabetologia. 1998;41(12):1532–3.
Esmatjes E, Rodriguez-Villar C, Ricart MJ, Casamitjana R, Martorell J, Sabater L, et al. Recurrence of immunological markers for type 1 (insulin-dependent) diabetes mellitus in immunosuppressed patients after pancreas transplantation. Transplantation. 1998;66(1):128–31.
Thivolet C, Abou-Amara S, Martin X, Lefrancois N, Petruzzo P, McGregor B, et al. Serological markers of recurrent beta cell destruction in diabetic patients undergoing pancreatic transplantation. Transplantation. 2000;69(1):99–103.
Petruzzo P, Andreelli F, McGregor B, Lefrancois N, Dawahra M, Feitosa LC, et al. Evidence of recurrent type I diabetes following HLA-mismatched pancreas transplantation. Diabetes Metab. 2000;26(3):215–8.
Braghi S, Bonifacio E, Secchi A, Di CV, Pozza G, Bosi E. Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with type 1 diabetes. Diabetes. 2000;49(2):218–24.
Ishida-Oku M, Iwase M, Sugitani A, Masutani K, Kitada H, Tanaka M, et al. A case of recurrent type 1 diabetes mellitus with insulitis of transplanted pancreas in simultaneous pancreas-kidney transplantation from cardiac death donor. Diabetologia. 2010;53(2):341–5.
Assalino M, Genevay M, Morel P, Demuylder-Mischler S, Toso C, Berney T. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation in the absence of GAD and IA-2 autoantibodies. Am J Transplant. 2012;12(2):492–5.
Occhipinti M, Lampasona V, Vistoli F, Bazzigaluppi E, Scavini M, Boggi U, et al. Zinc transporter 8 autoantibodies increase the predictive value of islet autoantibodies for function loss of technically successful solitary pancreas transplant. Transplantation. 2011;92(6):674–7.
Martins LS, Henriques AC, Fonseca IM, Rodrigues AS, Oliverira JC, Dores JM, et al. Pancreatic autoantibodies after pancreas-kidney transplantation—do they matter? Clin Transpl. 2014;28(4):462–9. doi:10.1111/ctr.12337. This study also suggests that autoantibodies are important for diabetes recurrence in pancreas transplantation.
Sutherland DE, Gruessner RW, Gruessner AC. Pancreas transplantation for treatment of diabetes mellitus. World J Surg. 2001;25(4):487–96.
Burke III GW, Vendrame F, Pileggi A, Ciancio G, Reijonen H, Pugliese A. Recurrence of autoimmunity following pancreas transplantation. Curr Diab Rep. 2011;11:413–9.
Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59(4):947–57.
Bashir SJ, Maibach HI. Alefacept (Biogen). Curr Opin Investig Drugs. 2001;2(5):631–4.
Sugiyama H, McCormick TS, Cooper KD, Korman NJ. Alefacept in the treatment of psoriasis. Clin Dermatol. 2008;26(5):503–8.
Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015. doi:10.1172/jci81722. This study reports the effects of an anti-memory cell agent in T1D for up to 2 years.
Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomized, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):284–94. doi:10.1016/s2213-8587(13)70111-6.
Krogvold L, Edwin B, Buanes T, Ludvigsson J, Korsgren O, Hyoty H, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014. doi:10.1007/s00125-013-3155-y. This study reports findings of pancreas biopsies obtained from patients with recent-onset T1D.
Bottazzo GF, Dean BM, McNally JM, Mackay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313(6):353–60.
Pugliese A, Yang M, Kusmarteva I, Heiple T, Vendrame F, Wasserfall C, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15(1):1–9.
Klinke DJ. Age-corrected beta cell mass following onset of type 1 diabetes mellitus correlates with plasma C-peptide in humans. PLoS One. 2011;6(11), e26873.
Klinke DJ. Extent of beta cell destruction is important but insufficient to predict the onset of type 1 diabetes mellitus. PLoS ONE. 2008;3(1), e1374.
Pugliese A, Vendrame F, Reijonen H, Atkinson MA, Campbell-Thompson M, Burke GW. New insight on human type 1 diabetes biology: nPOD and nPOD-transplantation. Current Diabetes Reports. 2014;14(10):530. doi:10.1007/s11892-014-0530-0.
Martin-Pagola A, Sisino G, Allende G, Dominguez-Bendala J, Gianani R, Reijonen H, et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia. 2008;51(10):1803–13.
Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol. 2000;20(3):900–11.
Tanaka S, Kobayashi T, Nakanishi K, Okubo M, Murase T, Hashimoto M, et al. Evidence of primary beta-cell destruction by T-cells and beta-cell differentiation from pancreatic ductal cells in diabetes associated with active autoimmune chronic pancreatitis. Diabetes Care. 2001;24(9):1661–7.
Vendrame F, Hopfner Y-Y, Diamantopoulos S, Virdi SK, Allende G, Snowhite IV, et al. Risk factors for type 1 diabetes recurrence in immunosuppressed recipients of simultaneous pancreas-kidney transplants. Am J Transplant. 2015. doi:10.1111/ajt.13426. This is the largest series of pancreas transplant recipients with thorough evaluation of autoantibodies and clinical history over more than 20 years, which defines risk factors for T1DR.
Sageshima J, Ciancio G, Gaynor JJ, Chen L, Guerra G, Kupin W, et al. Addition of anti-CD25 to thymoglobulin for induction therapy: delayed return of peripheral blood CD25-positive population. Clin Transplant. 2011;25(2):E132–E5.
Laughlin E, Burke G, Pugliese A, Falk B, Nepom G. Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol. 2008;128(1):23–30.
Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–9.
Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(1):a007732.
Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9(9):513–21.
Fisher MM, Watkins RA, Blum J, Evans-Molina C, Chalasani N, DiMeglio LA, et al. Elevations in circulating methylated and unmethylated preproinsulin DNA in new-onset type 1 diabetes. Diabetes. 2015. doi:10.2337/db15-0430.
Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, et al. Beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125(3):1163–73. doi:10.1172/jci78142.
Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, Benoist C, et al. Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc Natl Acad Sci U S A. 2015;112(7):2139–44. doi:10.1073/pnas.1424993112.
Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121(1):442–5.
Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308(22):2337–9.
Williams AJ, Thrower SL, Sequeiros IM, Ward A, Bickerton AS, Triay JM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97(11):E2109–13. doi:10.1210/jc.2012-1815.
Bayer AL, Pugliese A, Malek TR. The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation. Immunol Res. 2013;57(1–3):197–209.
Acknowledgments
Studies by the authors reviewed here were supported by grants from the National Institutes of Health (R01 DK070011, R01 DK052068), the JDRF (17-2011-594, 17-2012-3), the American Diabetes Association (RA-1-09-RA-413), the John C. Hench Foundation, and the Diabetes Research Institute Foundation, Hollywood, Florida. We are indebted to our research nurses, Lissett Tueros, Lois Hanson, and Sandra Flores.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
George W. Burke, III; Francesco Vendrame; Sahil K. Virdi; G. Ciancio; Linda Chen; Phillip Ruiz; Shari Messinger; Helena K. Reijonen; and Alberto Pugliese declare that they have no conflict of interest.
Ethical Approval
Studies described in this article involve human subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Our studies were approved by the University of Miami Institutional Review Board.
Informed Consent
Informed consent was obtained from all individuals who actively participated in the study. For some patients who did not actively participated in the study but whose data were retrospectively analyzed, studies were conducted under waiver of consent.
Ethical Approval and Animal Welfare
For studies described that involved experimental animals, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
This article is part of the Topical Collection on Treatment of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Burke, G.W., Vendrame, F., Virdi, S.K. et al. Lessons From Pancreas Transplantation in Type 1 Diabetes: Recurrence of Islet Autoimmunity. Curr Diab Rep 15, 121 (2015). https://doi.org/10.1007/s11892-015-0691-5
Published:
DOI: https://doi.org/10.1007/s11892-015-0691-5