Abstract
Purpose of Review
In the current era of implantable cardioverter-defibrillator procedures, the decision of whether or not to perform defibrillation threshold testing at the time of implantation is now less of a clinical conundrum. In this paper, we summarize this current practice, beginning with the physiology of defibrillation, followed by a review of the salient data, and discussion of specific situations where defibrillation threshold testing remains a clinical consideration.
Recent Findings
Two prospective randomized trials demonstrated no mortality difference and no overall complication rate difference between patients who underwent defibrillation testing at implant compared with patients who underwent no defibrillation testing.
Summary
Current recommendations support eliminating routine defibrillation testing in left-sided transvenous implantable cardioverter-defibrillator primary prevention implants. Defibrillation testing remains indicated in subcutaneous defibrillator implants in the absence of contraindications.


Similar content being viewed by others
Abbreviations
- CHD:
-
Congenital heart disease
- CRT:
-
Cardiac resynchronization therapy
- DFT:
-
Defibrillation threshold testing
- DT:
-
Defibrillation testing
- ICD:
-
Implantable cardioverter defibrillator
- LVEF:
-
Left ventricular ejection fraction
- NCDR:
-
National Cardiovascular Data Registry
- VF:
-
Ventricular fibrillation
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bigger JT, for the Coronary Artery Bypass Graft (Cabg) Patch Trial Investigators. N Engl J Med. 1997;337:1569–75.
Zipes DP, Fischer J, King RM, et al. Termination of ventricular fibrillation in dogs by depolarizing a critical amount of myocardium. Am J Cardiol. 1975;36:37–44.
Chen PS, Shibata N, Dixon EG, et al. Activation during ventricular defibrillation in open-chest dogs. Evidence of complete cessation and regeneration of ventricular fibrillation after unsuccessful shocks. J Clin Investig. 1986;77:810–23.
Mower MM, Mirowski M, Spear JF, et al. Patterns of ventricular activity during catheter defibrillation. Circulation. 1974;49:858–61.
• Mirowski M, Mower M, Gott VL, et al. Feasibility and effectiveness of low-energy defibrillation in man. Circulation. 1973;49:79–85. The 1973 original series describing catheter-based transvenous defibrillation in humans.
Dudel J. Elektrophysiologische Grundlagen der Defibrillation und künstlichen Stimulation des Herzens. Med Klin. 1968;63:2089–91.
Lermann BB, DiMaro JP, Haines DE. Current-based versus energy-based ventricular defibrillation: a prospective study. J Am Coll Cardiol. 1988;12:1259–64.
Dosdall DJ, Vladimir G, Fast VG, et al. Mechanisms of defibrillation. Annu Rev Biomed Eng. 2010;12:233–58.
Trayanova N, Roth BJ, Malden LJ. The responses of a spherical heart to a uniform electric field: A biodomain analysis of cardiac stimulation. IEEE Trans. Biomed. Eng. 1993;40:899–908.
Saksena S, An H, Mehra R, et al. Prospective comparison of biphasic and monophasic shocks for implantable cardioverter-defibrillators using endocardial leads. Am J Cardiol. 1992;70(3):304–10.
Strickberger SA, Daoud E, Goyal R, et al. Prospective randomized comparison of anodal monophasic shocks versus biphasic cathodal shocks on defibrillation energy requirements. Am Heart J. 1996;131(5):961–5.
Efimov IR, Cheng Y, Van Wagoner D, et al. Virtual electrode-induced phase singularity: a basic mechanism of defibrillation failure. Circ Res. 1998;82:918–25.
Efimov IR, Cheng Y, Yamanouchi Y, et al. Direct evidence of the role of virtual electrode induced phase singularity in success and failure of defibrillation. J Cardiovasc Electrophysiol. 2000;11:861–8.
• Bardy GH, Gliner BE, Kudenchuk P, et al. Truncated biphasic pulses for transthoracic defibrillation. Circulation. 1995;91(6):1768–74. This prospective multicenter study demonstrated similar effectiveness of truncated biphasic pulses compared to monophasic damped sine wave pulses for defibrillation efficacy with lower energy and voltage requirements.
Gold MR, Kavesh NG, Peters RW, Shorofsky SR. Biphasic waveforms prevent the chronic rise of defibrillation thresholds with a transvenous lead system. J Am Coll Cardiol. 1997;30:233–6.
Poole J, Bardy G, Dolack G, et al. Serial defibrillation thresholds in man: a prospective controlled study. J Cardiovasc Electrophysiol. 1995;6:19–25.
Mann DE, Klein RC, Higgins SL, LESS Investigators, et al. The Low Energy Safety Study (LESS): rationale, design, patient characteristics, and device utilization. Am Heart J. 2002;143(2):199–204.
Strickberger SA, Doud EG, Davidson T, et al. Probability of successful defibrillation at multiples of the defibrillation energy requirements in patients with an implantable defibrillator. Circulation. 1997;96:1217–23.
Tokano T, Pelosi F, Flemming M, et al. Long-term evaluation of the ventricular defibrillation energy requirement. J Cardiovasc Electrophysiol. 1998;9:916–20.
Swerdlow CD, Kass RM, O'Connor ME, et al. Effect of shock waveform on relationship between upper limit of vulnerability and defibrillation threshold. J Cardiovasc Electrophysiol. 1998;9(4):339–49.
Swerdlow CD, Sheata M, Chen PS. Using the upper limit of vulnerability to assess defibrillation efficacy at implantation of ICDs. PACE. 2007;30(5):675–0.
• Gold MR, Higgins S, Klein R, et al. Efficacy and temporal stability of reduced safety margins for ventricular defibrillation: primary results from the Low Energy Safety Study (LESS). Circulation. 2002;105(17):2043–8. The results of the Low Energy Safety Study demonstrated that a 5-J biphasic safety margin was safe for conversion of spontaneously ventricular arrhythmias with a rate > 200 bpm. The authors used a pre-specified modified step-down algorithm for defibrillation testing.
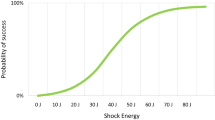
• Jones DL, Irish WD, Klein GJ. Defibrillation efficacy comparison of defibrillation threshold versus dose-response curve determination. Circ Res. 1991;69:45–51. Elegant animal study describing the dose-response relationship of defibrillation energy.
Reynolds CR, Nikolski V, Sturdivant JL, et al. Randomized comparison of defibrillation thresholds from the right ventricular apex and outflow tract. Heart Rhythm. 2010;7:1561–6.
Larsen JM, Hjortshoj SP, Nielsen JC, et al. Single-coil and dual-coil defibrillator leads and association with clinical outcomes in a complete Danish nationwide ICD cohort. Heart Rhythm. 2016;13:706–12.
Kroll MW, Efimov IR, Tchou PJ. Present understanding of shock polarity for internal defibrillation: the obvious and nonobvious clinical implications. Pacing Clin Electrophysiol. 2006;29:885–91.
Aoukar PS, Poole JE, Johnson GW, et al. No benefit of a dual coil over a single coil ICD lead: evidence from the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm. 2013;10:970–6.
Baccillieri MS, Gasparini G, Benacchi L, et al. Multicentre comparison Of shock efficacy using single vs. Dual-coil lead systems and Anodal vs. cathodal polarITY defibrillation in patients undergoing transvenous cardioverter-defibrillator implantation. The MODALITY study. J Interv Car Electrophysiol. 2015;43:45–54.
The Antiarrhythmics versus Implantable Defibrillator (AVID) Investigators. A comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83.
•• Bardy GH, Lee KL, Mark DB, for the Sudden Cardiac Death in Heart Failure Trial Investigators, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. SCD-HeFT is the largest randomized placebo controlled ICD trial in ischemic and nonischemic cardiomyopathy patients with mild to moderate heart failure. This seminal trial demonstrated total mortality benefit with ICD implantation.
Bianchi S, Ricci RP, Gasparini M, et al. Defibrillation testing during implantable cardioverter defibrillator implantation in Italian current practice: the Assessment of Long-term Induction clinical ValuE (ALIVE) project. Am Heart J. 2011;162:390–7.
Moss AJ, Brown MW, Cannon DS, et al. Multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT): design and clinical protocol. Ann Noninvasive Electrocardiol. 2005;10(4 Suppl):34–43.
Aktas MK, Huang DT, Daubert JP, et al. Effect of defibrillation threshold testing on heart failure hospitalization or death in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Heart Rhythm. 2013;10:193–9.
Blatt JA, Poole JE, Johnson GW, for the SCD-HeFT Investigators, et al. No benefit for defibrillation threshold testing in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol. 2008;52:551–6.
Michowitz Y, Lellouche N, Contractir T, et al. Defibrillation threshold testing fails to show clinical benefit during long-term follow-up of patients undergoing cardiac resynchronization therapy defibrillator implantation. Europace. 2011;13:683–8.
Chung MK, Holcomb RG, Mittal S, for the REPLACE investigators, et al. REPLACE DARE (Death After Replacement Evaluation) score determinants of all-cause mortality after implantable device replacement or upgrade from the REPLACE registry. Circ Arrhythm Electrophysiol. 2014;7:1048–56.
•• Healey JS, Hohnloser SH, Glickson M, on behalf of the Shockless IMPLant Evaluation [SIMPLE] investigators, et al. Cardioverter defibrillator implantation without induction of ventricular fibrillation: a single-blind, non-inferiority, randomized controlled trial. Lancet. 2015;385:785–91. The SIMPLE ICD trial was a single-blind multicenter non-inferiority trial of defibrillation testing in de novo left-sided ICD implants, and demonstrated no benefit on first shock efficacy nor arrhythmic death.
•• Bansch D, Bonnemeier H, Brandt J, for the NORDIC ICD Trial Investigators, et al. Eur Heart J. 2015;36:2500–7. The NORDIC trial was a prospective randomized parallel group non-inferiority trial of defibrillation threshold testing at time of ICD implant. This demonstrated no mortality benefit from such testing and did not reduce first shock efficacy.
Phan K, Ha H, Kabunga P, et al. Systematic review of defibrillation threshold testing at de novo implantation. Circ Arrhythm Electrophysiol. 2016;9:e003357. https://doi.org/10.1161/CIRCEP.115.003357.
Bonanno C, Rossillo A, Paccanaro M, et al. A meta-analysis of randomized trials comparing the safety and efficacy of intraoperative defibrillation testing with no defibrillation testing on ICD implantation. J Am Coll Cardiol: Clin Electrophysiol. 2017;3(8):917–9.
Birnie D, Tung S, Simpson C, et al. Complications associated with defibrillation threshold testing: the Canadian experience. Heart Rhythm. 2008;5:387–90.
Hsu JC, Marcus GM, Al-Khatib SM, et al. Predictors of an inadequate defibrillation safety margin at ICD implantation: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2014;64:256–64.
Russo AM, Sauer W, Gerstenfeld EP, et al. Defibrillation threshold testing: is it really necessary at the time of implantable cardioverter-defibrillator insertion? Heart Rhythm. 2005;2:456–61.
Epstein A, Ellenbogen K, Kirk K. Clinical characteristics and outcomes of patients with high defibrillation thresholds: a multicenter study. Circulation. 1992;86:1206–16.
Shukla HH, Flaker GC, Jayam V, et al. High defibrillation thresholds in transvenous biphasic implantable defibrillators: clinical predictors and prognostic implications. PACE. 2003;26:44–8.
• Hohnloser SH, Dorian P, Roberts R, et al. Effect of amiodarone and sotalol on ventricular defibrillation threshold: the optimal pharmacologic therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation. 2006;114:104–9. This randomized trial of sotalol, beta blocker, or amiodarone determined that the reassessment of the DFT after anti-arrhythmic drug initiation was not routinely required.
Stevens SK, Haffajee CI, Naccarelli GV, et al. Effects of oral propafenone on defibrillation and pacing thresholds in patients receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 1996;28:418–22.
Kirk MM, Shorofosky SR, Gold MR. Comparison of the effects of active left and right pectoral pulse generators on defibrillation efficacy. Am J Cardiol. 2001;88:1308–11.
Gold MR, Shis HT, Herre J, for the Low Energy Safety Study Investigators, et al. Comparison of defibrillation efficacy and survival associated with right versus left pectoral placement for implantable defibrillators. Am J Cardiol. 2007;100:243–6.
Parkash R, Thibault B, Mangat I, et al. Canadian registry of implantable electronic device outcomes: surveillance of the Riata lead under advisory. Circ Arrhythm Electrophysiol. 2016;9:e004282. https://doi.org/10.1161/CIRCEP116.004282.
Hauser RG, Maisal WH, Friedman PA, et al. Longevity of Sprint Fidelis implantable cardioverter-defibrillator leads and risk factors for failure: implications for patient management. Circulation. 2011;123:358–63.
Leong DP, Van Erven L. Unrecognized failure of a narrow caliber defibrillation lead: the role of defibrillation threshold testing in identifying an unprotected individual. PACE. 2012;35:e154–5.
Bun SS, Duytschaever M, Tavernier R. Defibrillation testing can reveal ‘concealed’ lead fracture. Europace. 2012;15:54. https://doi.org/10.1093/Europace/eus173.
Ruetz LL, Koehler JL, Brown ML, et al. Sinus rhythm R-wave amplitude as a predictor of ventricular fibrillation undersensing in patients with implantable cardioverter-defibrillator. Heart Rhythm. 2015;12:2411–8.
Almiquist AK, Montgomery JV, Haas TS, et al. Cardioverter-defibrillator implantation in high-risk patients with hypertrophic cardiomyopathy. Heart Rhythm. 2005;2:814–9.
• Gleva MJ, Wang Y, Curtis JP, et al. Complications associated with implantable cardioverter defibrillators in adults with congenital heart disease or left ventricular non-compaction cardiomyopathy (from the NCDR ICD Registry). Am J Cardiol. 2017;120:1891–8. This is the largest series of ICD implantations in adults with congenital heart disease or left ventricular non-compaction cardiomyopathy.
Wilkoff BL, Fauchier L, Stiles MK, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13(2):e50–76.
Lambiase PD, Barr C, Theuns DAMJ, on behalf of the EFFORTLESS Investigators, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–65. https://doi.org/10.1093/eurheartj/ehu112.
Heist EK, Belalcazar A, Wtahl W, et al. Determinants of subcuatenous implantable cardioverter-defibrillator efficacy. JACC Clin Electrophysiol. 2017;3(4):405–15.
Bennett M, Parkash R, Nery P, et al. Canadian Cardiovascular Society/Canadian Heart Rhythm Society 2016 Implantable Cardioverter–Defibrillator Guidelines. Can J Cardiol. 2017;33:174–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Marye J. Gleva reports that Washington University in St. Louis participated in some of the randomized trials of implantable cardioverter defibrillators that are included in the references.
Melissa Robinson reports personal fees from Medtronic, Abbott, and Boston Scientific.
Jeanne Poole reports personal fees from Medtronic, Kestra Inc., Boston Scientific, and Biotronik.
Marye J. Gleva reports personal fees from Abbott, BIOTRONIK, Boston Scientific, Kestra, Inc, Medtronic, and Zoll Medical
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Invasive Electrophysiology and Pacing
Rights and permissions
About this article
Cite this article
Gleva, M.J., Robinson, M. & Poole, J. The Saga of Defibrillation Testing: When Less Is More. Curr Cardiol Rep 20, 44 (2018). https://doi.org/10.1007/s11886-018-0987-6
Published:
DOI: https://doi.org/10.1007/s11886-018-0987-6