Abstract
Purpose of Review
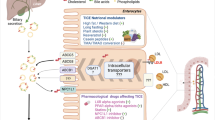
Transintestinal cholesterol excretion (TICE) is a non-biliary pathway that excretes excess cholesterol from the body through feces. This article focuses on the research progress of the TICE pathway in the last few years, including the discovery process of the TICE pathway, its molecular mechanism, and potential clinical applications.
Recent Findings
Cholesterol homeostasis is vital for cardiovascular diseases, stroke, and neurodegenerative diseases. Beyond the cholesterol excretion via hepatobiliary pathway, TICE contributes significantly to reverse cholesterol transport ex vivo and in vivo. Nuclear receptors are ligand-activated transcription factors that regulate cholesterol metabolism. The farnesoid X receptor (FXR) and liver X receptor (LXR) activated, respectively, by oxysterols and bile acids promote intestinal cholesterol secretion through ABCG5/G8. Nutrient regulators and intestinal flora also modulate cholesterol secretion through the TICE pathway. TICE allows direct elimination of plasma cholesterol, which may provide an attractive therapeutic targets.
Summary
TICE pathway may provide a potential target to stimulate cholesterol elimination and reduce the risk of cardiovascular diseases.




Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hu J, et al. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond). 2010;7:47.
Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):13–22.
Glomset JA, Norum KR. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65.
Garçon D, et al. Transintestinal cholesterol excretion in health and disease. Curr Atheroscler Rep. 2022;24(3):153–60.
Stellaard, F. From dietary cholesterol to blood cholesterol, physiological lipid fluxes, and cholesterol homeostasis. Nutrients. 2022; 14(8).
de Boer JF, et al. Intestinal Farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology. 2017;152(5):1126-1138.e6.
Tanaka Y, Kamisako T. Regulation of the expression of cholesterol transporters by lipid-lowering drugs ezetimibe and pemafibrate in rat liver and intestine. Biochim Biophys Acta Mol Basis Dis. 2021;1867(11):166215.
•• Luo, J., H. Yang, B.L. Song. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol, 2020. 21(4): 225–245. The study identified the key factors that govern the four pathways of cholesterol biosynthesis, uptake, export and esterification, and the main mechanisms by which they respond to different sterol levels.
Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport. Circ Res. 2019;124(10):1505–18.
Goedeke L, Fernández-Hernando C. Regulation of cholesterol homeostasis. Cell Mol Life Sci. 2012;69(6):915–30.
Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96(20):11041–8.
Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46.
Sun LP, et al. Insig required for sterol-mediated inhibition of Scap/SREBP binding to COPII proteins in vitro. J Biol Chem. 2005;280(28):26483–90.
Sever N, et al. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol Cell. 2003;11(1):25–33.
Goldstein JL, Rawson RB, Brown MS. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys. 2002;397(2):139–48.
Altmann SW, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–4.
van Heek M, et al. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol. 2001;134(2):409–17.
Garcia-Calvo M, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci U S A. 2005;102(23):8132–7.
Altmann SW, et al. The identification of intestinal scavenger receptor class B, type I (SR-BI) by expression cloning and its role in cholesterol absorption. Biochim Biophys Acta. 2002;1580(1):77–93.
Li PS, et al. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat Med. 2014;20(1):80–6.
Johnson TA, Pfeffer SR. Ezetimibe-sensitive cholesterol uptake by NPC1L1 protein does not require endocytosis. Mol Biol Cell. 2016;27(11):1845–52.
Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011;73:239–59.
Anderson RA, et al. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem. 1998;273(41):26747–54.
Ingram MF, Shelness GS. Folding of the amino-terminal domain of apolipoprotein B initiates microsomal triglyceride transfer protein-dependent lipid transfer to nascent very low density lipoprotein. J Biol Chem. 1997;272(15):10279–86.
Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290(5497):1771–5.
Lee MH, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27(1):79–83.
Yu L, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99(25):16237–42.
Yu L, et al. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110(5):671–80.
Temel RE, et al. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res. 2005;46(11):2423–31.
Brunham LR, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116(4):1052–62.
Tang W, et al. Niemann-Pick C1-like 1 is required for an LXR agonist to raise plasma HDL cholesterol in mice. Arterioscler Thromb Vasc Biol. 2008;28(3):448–54.
Doonan LM, Fisher EA, Brodsky JL. Can modulators of apolipoproteinB biogenesis serve as an alternate target for cholesterol-lowering drugs? Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(7):762–71.
Yu L, et al. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J Biol Chem. 2005;280(10):8742–7.
Cheng SH, Stanley MM. Secretion of cholesterol by intestinal mucosa in patients with complete common bile duct obstruction. Proc Soc Exp Biol Med. 1959;101(2):223–5.
van der Velde AE, et al. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133(3):967–75.
Smit JJ, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75(3):451–62.
Brown JM, et al. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283(16):10522–34.
Simmonds WJ, Hofmann AF, Theodor E. Absorption of cholesterol from a micellar solution: intestinal perfusion studies in man. J Clin Invest. 1967;46(5):874–90.
Le May C, et al. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler Thromb Vasc Biol. 2013;33(7):1484–93.
Jakulj L, et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 2016;24(6):783–94.
Moreau F, et al. In vivo evidence for transintestinal cholesterol efflux in patients with complete common bile duct obstruction. J Clin Lipidol. 2019;13(1):213-217.e1.
Hirata T, et al. Molecular mechanisms of subcellular localization of ABCG5 and ABCG8. Biosci Biotechnol Biochem. 2009;73(3):619–26.
Lu K, et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. 2001;69(2):278–90.
Sabeva NS, Rouse EJ, Graf GA. Defects in the leptin axis reduce abundance of the ABCG5-ABCG8 sterol transporter in liver. J Biol Chem. 2007;282(31):22397–405.
Langheim S, et al. ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J Lipid Res. 2005;46(8):1732–8.
Wang J, et al. Relative roles of ABCG5/ABCG8 in liver and intestine. J Lipid Res. 2015;56(2):319–30.
Brown JM, Yu L. Opposing gatekeepers of apical sterol transport: Niemann-Pick C1-like 1 (NPC1L1) and ATP-binding cassette transporters G5 and G8 (ABCG5/ABCG8). Immunol Endocr Metab Agents Med Chem. 2009;9(1):18–29.
Jakulj L, et al. Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett. 2010;584(16):3625–8.
van der Velde AE, et al. Regulation of direct transintestinal cholesterol excretion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G203-g208.
Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–25.
•• Blankestijn, M., et al., Induction of fecal cholesterol excretion is not effective for the treatment of hyperbilirubinemia in Gunn rats. Pediatr Res. 2021; 89(3): 510–517. Findings from this study suggest that OCA enhanced TICE by activating FXR.
Janowski BA, et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383(6602):728–31.
Plōsch T, et al. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J Biol Chem. 2002;277(37):33870–7.
Repa JJ, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289(5484):1524–9.
Kruit JK, et al. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 2005;128(1):147–56.
van der Veen JN, et al. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284(29):19211–9.
• Lifsey HC et al. Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine. J NutrBiochem, 2020; 76: 108263. This study shows that LXR agonist T0901317 stimulates the TICE pathway by increasing the expression of ABCG5 and ABCG8 in the liver and duodenum.
Grefhorst A, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277(37):34182–90.
van der Veen JN, et al. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res. 2005;46(3):526–34.
Oliver WR Jr, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98(9):5306–11.
Sprecher DL, et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler Thromb Vasc Biol. 2007;27(2):359–65.
Zhang DW, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282(25):18602–12.
Sachdev V, et al. Novel role of a triglyceride-synthesizing enzyme: DGAT1 at the crossroad between triglyceride and cholesterol metabolism. Biochim Biophys Acta. 2016;1861(9 Pt A):1132–41.
Marshall SM, et al. Acute sterol o-acyltransferase 2 (SOAT2) knockdown rapidly mobilizes hepatic cholesterol for fecal excretion. PLoS One. 2014;9(6):e98953.
Sokolović M, et al. Unexpected effects of fasting on murine lipid homeostasis–transcriptomic and lipid profiling. J Hepatol. 2010;52(5):737–44.
Calpe-Berdiel L, Escolà-Gil JC, Blanco-Vaca F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 2009;203(1):18–31.
Brufau G, et al. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS One. 2011;6(6):e21576.
Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85.
Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63.
Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol. 2008;28(7):1296–7.
Warrier M, et al. The TMAO-Generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10(3):326–38.
Vrins CL, et al. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J Lipid Res. 2009;50(10):2046–54.
Chandak PG, et al. Lack of acyl-CoA:diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice. Biochim Biophys Acta. 2011;1811(12):1011–20.
Funding
This work was supported by the Natural Science Foundation of Henan Province (grant number 182300410316, Y.Z.), the Key Program for Science and Technology of Henan Province (grant number 202102310808, W.L.), the Key Program for Science and Technology of Henan Province (grant number 222102310578, P.L.), and the Co-constructed Program for Medical Science and Technology of Henan Province (grant numbers LHGJ20191297, P.L.).
Author information
Authors and Affiliations
Contributions
Huimin Xu drafted the article. Yiyang Xin conducted the figures. Jiaxin Wang and Zixin Liu reviewed the article. Yutong Cao and Weiguo Li revised the article. Peng Liu, Yandong Wang, and Yun Zhou guided the writing.
Corresponding authors
Ethics declarations
Consent to Participate
The authors consented to participate.
Consent to Publish
The authors agreed to publish this paper.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Xin, Y., Wang, J. et al. The TICE Pathway: Mechanisms and Potential Clinical Applications. Curr Atheroscler Rep 25, 653–662 (2023). https://doi.org/10.1007/s11883-023-01147-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01147-6