Abstract
Purpose of Review
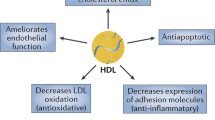
High-density lipoproteins (HDL) are thought to exert a protective role against atherosclerosis. The measurement of the cholesterol mass within HDL (HDL-C) represents a good biomarker of cardiovascular health, but HDL-C appears to be a poor therapeutic target. Here, we discuss new targets for the development of HDL-directed therapies.
Recent Findings
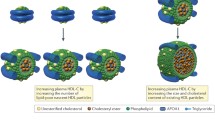
Among cardio-protective functions of HDL particles, the ability of HDL to remove cholesterol from cells involved in the early stages of atherosclerosis is considered one of the most important functions. This process, termed “HDL biogenesis,” is initiated by the formation of highly specialized plasma membrane micro-domains by the ATP-binding cassette transporter A1 (ABCA1) and the binding of apolipoproteins (apo) such as apoA-I, the major protein moiety of HDL, to the micro-domains. Although early strategies aimed at increasing HDL biogenesis by upregulating ABCA1 or apoA-I gene expression have not met with clinical success, recent advances in understanding transcriptional, post-transcriptional, and post-translational regulatory pathways propose new targets for the promotion of HDL biogenesis. We have recently reported that a novel apoA-I-binding protein desmocollin 1 (DSC1) prevents HDL biogenesis and that inhibition of apoA-I-DSC1 interactions promotes HDL biogenesis by stabilizing ABCA1. This new HDL regulation pathway nominates DSC1 as an attractive pharmacological target.
Summary
In the absence of clinically useful therapy to increase HDL biogenesis, finding novel targets to unlock the therapeutic potential of HDL is highly desired. Modulation of apoA-I-DSC1 interactions may be a viable strategy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80.
Ko DT, Alter DA, Guo H, Koh M, Lau G, Austin PC, et al. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol. 2016;68(19):2073–83.
• Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38:2478–86. Demonstrate complex associations between HDL cholesterol levels and cardiovascular disease and mortality.
Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128(14):1504–12.
Kingwell BA, Chapman MJ, Kontush A, Miller NE. HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov. 2014;13(6):445–64.
Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933–42.
Luscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res. 2014;114(1):171–82.
• Choi HY, Hafiane A, Schwertani A, Genest J. High-density lipoproteins: biology, epidemiology, and clinical management. Can J Cardiol. 2017;33(3):325–33. Athero-protective HDL functionality and therapeutic potential of HDL are reviewed.
Marz W, Kleber ME, Scharnagl H, Speer T, Zewinger S, Ritsch A, et al. HDL cholesterol: reappraisal of its clinical relevance. Clin Res Cardiol. 2017; https://doi.org/10.1007/s00392-017-1106-1.
Hafiane A, Genest J. HDL, atherosclerosis, and emerging therapies. Cholesterol. 2013;2013:891403.
Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res. 2015;56(8):1519–30.
Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res. 2013;54(11):2950–63.
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–93.
Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, et al. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Circulation. 2017;135(25):2494–504.
•• Qian H, Zhao X, Cao P, Lei J, Yan N, Gong X. Structure of the human lipid exporter ABCA1. Cell. 2017;169(7):1228–39 e10. Cryo-electron microscopy structure of the human ABCA1 provides insight into the action mechanism of ABCA1.
Iatan I, Bailey D, Ruel I, Hafiane A, Campbell S, Krimbou L, et al. Membrane microdomains modulate oligomeric ABCA1 function: impact on apoAI-mediated lipid removal and phosphatidylcholine biosynthesis. J Lipid Res. 2011;52(11):2043–55.
Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289(35):24020–9.
• Segrest JP, Jones MK, Catte A, Manchekar M, Datta G, Zhang L, et al. Surface density-induced pleating of a lipid monolayer drives nascent high-density lipoprotein assembly. Structure. 2015;23(7):1214–26. Propose a mechanism for the creation of specialized plasma membrane micro-domains required for the assembly of HDL.
Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125(15):1905–19.
Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353(6341):265–7.
Paszty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94(2):899–903.
Zannis VI, Duka A, Drosatos K, Sanoudou D, Koukos G, Zanni E, et al. Regulation of apoA-I gene expression and prospects to increase plasma apoA-I and HDL levels. In: Schaefer EJ, editor. High density lipoproteins, dyslipidemia, and coronary heart disease. New York: Springer; 2010. p. 15–24.
Staels B, Auwerx J. Role of PPAR in the pharmacological regulation of lipoprotein metabolism by fibrates and thiazolidinediones. Curr Pharm Des. 1997;3:1–14.
Khera AV, Millar JS, Ruotolo G, Wang MD, Rader DJ. Potent peroxisome proliferator-activated receptor-alpha agonist treatment increases cholesterol efflux capacity in humans with the metabolic syndrome. Eur Heart J. 2015;36(43):3020–2.
Guo Y, Fan Y, Zhang J, Lomberk GA, Zhou Z, Sun L, et al. Perhexiline activates KLF14 and reduces atherosclerosis by modulating apoA-I production. J Clin Invest. 2015;125(10):3819–30.
Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci U S A. 2013;110(49):19754–9.
McLure KG, Gesner EM, Tsujikawa L, Kharenko OA, Attwell S, Campeau E, et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One. 2013;8(12):e83190.
Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114(11):1733–42.
Huang Y, DiDonato JA, Levison BS, Schmitt D, Li L, Wu Y, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med. 2014;20(2):193–203.
DiDonato JA, Huang Y, Aulak KS, Even-Or O, Gerstenecker G, Gogonea V, et al. Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation. 2013;128(15):1644–55.
Remaley AT, Stonik JA, Demosky SJ, Neufeld EB, Bocharov AV, Vishnyakova TG, et al. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280(3):818–23.
Hara H, Hara H, Komaba A, Yokoyama S. Alpha-helical requirements for free apolipoproteins to generate HDL and to induce cellular lipid efflux. Lipids. 1992;27(4):302–4.
Hafiane A, Genest J. ATP binding cassette A1 (ABCA1) mediates microparticle formation during high-density lipoprotein (HDL) biogenesis. Atherosclerosis. 2017;257:90–9.
Nandi S, Ma L, Denis M, Karwatsky J, Li Z, Jiang XC, et al. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. J Lipid Res. 2009;50(3):456–66.
Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275(36):28240–5.
Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97(22):12097–102.
Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13(6):433–44.
Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme a desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26(18):6786–98.
Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277(13):11019–25.
Talukdar S, Hillgartner FB. The mechanism mediating the activation of acetyl-coenzyme A carboxylase-alpha gene transcription by the liver X receptor agonist T0-901317. J Lipid Res. 2006;47(11):2451–61.
Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277(11):9520–8.
Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–30.
Hien HTM, Ha NC, Thom LT, Hong DD. Squalene promotes cholesterol homeostasis in macrophage and hepatocyte cells via activation of liver X receptor (LXR) alpha and beta. Biotechnol Lett. 2017;39:1101–7.
Canfran-Duque A, Lin CS, Goedeke L, Suarez Y, Fernandez-Hernando C. Micro-RNAs and high-density lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 2016;36(6):1076–84.
Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13(8):622–38.
Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328(5985):1566–9.
Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–3.
• Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161(1):161–72. Outstanding review on past, present, and future studies of cholesterol.
Ono K. Functions of microRNA-33a/b and microRNA therapeutics. J Cardiol. 2016;67(1):28–33.
Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–40.
Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101(31):11245–50.
Kimura Y, Tamasawa N, Matsumura K, Murakami H, Yamashita M, Matsuki K, et al. Clinical significance of determining plasma MicroRNA33b in type 2 diabetic patients with dyslipidemia. J Atheroscler Thromb. 2016;23(11):1276–85.
Munehira Y, Ohnishi T, Kawamoto S, Furuya A, Shitara K, Imamura M, et al. Alpha1-syntrophin modulates turnover of ABCA1. J Biol Chem. 2004;279(15):15091–5.
Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277(25):22426–9.
Lv YC, Yin K, Fu YC, Zhang DW, Chen WJ, Tang CK. Posttranscriptional regulation of ATP-binding cassette transporter A1 in lipid metabolism. DNA Cell Biol. 2013;32(7):348–58.
Wang L, Palme V, Rotter S, Schilcher N, Cukaj M, Wang D, et al. Piperine inhibits ABCA1 degradation and promotes cholesterol efflux from THP-1-derived macrophages. Mol Nutr Food Res. 2017;61(4).
Huang L, Fan B, Ma A, Shaul PW, Zhu H. Inhibition of ABCA1 protein degradation promotes HDL cholesterol efflux capacity and RCT and reduces atherosclerosis in mice. J Lipid Res. 2015;56(5):986–97.
Huang X, Dixit VM. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res. 2016;26(4):484–98.
Ogura M, Ayaori M, Terao Y, Hisada T, Iizuka M, Takiguchi S, et al. Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2011;31(9):1980–7.
Mizuno T, Hayashi H, Naoi S, Sugiyama Y. Ubiquitination is associated with lysosomal degradation of cell surface-resident ATP-binding cassette transporter A1 (ABCA1) through the endosomal sorting complex required for transport (ESCRT) pathway. Hepatology. 2011;54(2):631–43.
Hsieh V, Kim MJ, Gelissen IC, Brown AJ, Sandoval C, Hallab JC, et al. Cellular cholesterol regulates ubiquitination and degradation of the cholesterol export proteins ABCA1 and ABCG1. J Biol Chem. 2014;289(11):7524–36.
•• Choi HY, Ruel I, Malina A, Garrod DR, Oda MN, Pelletier J, et al. Desmocollin 1 is abundantly expressed in atherosclerosis and impairs high-density lipoprotein biogenesis. Eur Heart J. 2017; https://doi.org/10.1093/eurheartj/ehx340. Desmocollin 1 is identified as a novel apoA-I-binding protein involved in the formation of HDL.
Rajapaksha M, Kaur J, Bose M, Whittal RM, Bose HS. Cholesterol-mediated conformational changes in the steroidogenic acute regulatory protein are essential for steroidogenesis. Biochemistry. 2013;52(41):7242–53.
Grouleff J, Irudayam SJ, Skeby KK, Schiott B. The influence of cholesterol on membrane protein structure, function, and dynamics studied by molecular dynamics simulations. Biochim Biophys Acta. 2015;1848(9):1783–95.
Gao Y, Zhou Y, Goldstein JL, Brown MS, Radhakrishnan A. Cholesterol-induced conformation changes in the sterol-sensing domain of the scap protein suggest feedback mechanism to control cholesterol synthesis. J Biol Chem. 2017;292:8729–37.
Hafiane A, Genest J. High density lipoproteins: measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015;3:175–88.
Funding
This work was supported by a grant from the Canadian institutes of Health Research (CIHR MOP 15042 JG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jacques Genest and Hong Y. Choi declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nonstatin Drugs
Rights and permissions
About this article
Cite this article
Genest, J., Choi, H.Y. Novel Approaches for HDL-Directed Therapies. Curr Atheroscler Rep 19, 55 (2017). https://doi.org/10.1007/s11883-017-0699-1
Published:
DOI: https://doi.org/10.1007/s11883-017-0699-1