Abstract
Purpose
The privatization of space travel is opening civilian spaceflight to an unprecedented number of individuals now and in the immediate future. The increase in the number and diversity of space travelers will mean increased exposure to both physiologic and pathologic changes observed during acute and prolonged microgravity.
Aims
In this paper, we describe the anatomic, physiologic, and pharmacologic factors to consider that impact acute angle-closure glaucoma risk during spaceflight.
Conclusions
Based on these factors, we elaborate upon areas of medical considerations and provide future recommendations that may aid in reducing the risk of acute angle-closure glaucoma in the next era of spaceflight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The privatization of space travel is opening civilian spaceflight to an unprecedented number of individuals now and in the immediate future [1]. The increase in the number and diversity of space travelers will mean increased exposure to both physiologic and pathologic changes observed during acute and prolonged microgravity [2]. Rare terrestrial ocular emergency conditions including acute angle-closure glaucoma pose special and unique challenges in space flight. Chronic spaceflight exposure to radiation leading to radiation cataract is another potential risk for precipitating angle closure.
In this paper, we describe the anatomic, physiologic, and pharmacologic factors to consider that impact acute angle-closure glaucoma risk during spaceflight. Based on these factors, we elaborate upon areas of medical considerations and provide future recommendations that may aid in reducing the risk of acute angle-closure glaucoma in the next era of spaceflight.
Several physiological mechanisms have been proposed to explain ocular findings seen in spaceflight. The cephalad redistribution of bodily fluids, particularly the cephalad circulation of cerebral spinal fluid (CSF), that astronauts on the International Space Station (ISS) experience in a microgravity setting is a major factor. Numerous physiological and anatomical alterations are possibly caused by confinement into ocular interstitial spaces. Cerebral edema, a consequence of diverse types of brain tissue injuries, has been used as a paradigm to try to further understand the etiology of the neuro-ocular findings unique to spaceflight. These findings are collectively known as spaceflight associated neuro-ocular syndrome (SANS) and have been hypothesized to occur due to cephalad fluid shift [3, 4]. This constellation of neuro-ophthalmic findings have been noted to be one of the large physiologic barriers to exploration spaceflight such as the mission to Mars [5, 6]. Choroidal expansion occurring in SANS could potentially lead to AACG [7].
Spaceflight or simulated microgravity may also affect a number of other variables related to cerebral autoregulation, including decreases in blood volume and perhaps vascular characteristics [8]. The mechanism most often cited to explain orthostatic sensitivity after spaceflight is the decrease in cerebral blood flow autoregulation brought-on by the lack of the gravitational field [8]. In one rodent study, cerebral arteries demonstrated hypertrophy (media layer) in microgravity [9].
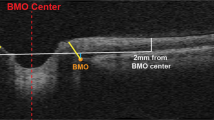
AACG occurs when aqueous humor outflow is interrupted in the trabecular meshwork, leading to an increase in intraocular pressure (Fig. 1). AACG can be divided into four subtypes: pupillary block, anterior lens subluxation, plateau iris syndrome, and crowded angle which will be described below.
a Anterior segment OCT of an angle closure glaucoma with narrow angles (circled). b Anterior segment OCT showing wide angles (circled). Both circles contain the trabecular meshwork. Reprinted with permission from Angmo D, Nongpiur ME, Sharma R, Sidhu T, Sihota R, and Dada T. Clinical utility of anterior segment swept-source optical coherence tomography in glaucoma. Oman J Ophthalmol. Under Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License
Pupillary block
The most frequent cause of AACG is called “pupillary block,” which develops when the pupillary portion of the iris and lens becomes functionally blocked between the anterior and posterior chambers [10]. Typically, the iris bends forward (iris bombe) as a result of increased intraocular pressure in the posterior chamber and accompany pupillary block [10].
Crowded angle
Patients with a crowded angle have thicker iris tissue at the base, leaving less room for the trabecular meshwork to drain. The iris is dragged peripherally and moved into the iridocorneal angle as a result of the contraction of the iris dilator muscles in mydriasis. Pupillary block often happens while the iris is returning to its resting undilated stage after being dilated, which might come from this crowding of tissue and obstruction of the trabecular meshwork [10].
Anterior lens subluxation
When the anterior lens subluxes, the lens protrudes and either contacts the iris or enters the anterior chamber. Because the zonules anchoring the lens in place are naturally prone to laxity, this might arise. Many conditions (e.g., pseudoexfoliation syndrome, Marfan syndrome, homocystinuria, Ehlers-Danlos syndrome, and Weill-Marchesani syndrome) can lead to zonular laxity, which may all affect ocular structures [10]. Similar to pupillary block, aqueous fluid is unable to drain via the trabecular meshwork because the anterior and posterior chambers are connected but blocked.
Plateau iris syndrome
An anterior chamber depth that is generally normal, a crowded angle, and a flat iris are all indicative of plateau iris syndrome (PIS). The peripheral iris base of the iris inserts more anteriorly on the ciliary body, resulting in PIS. The iridocorneal angle becomes smaller as a consequence, making it more likely to get blocked during mydriasis. Patients with PIS thus remain at risk for AACG despite having a patent iridotomy.
It is important to note that the greatest anatomical risk factor for angle closure is depth of the anterior chamber [11]. Other risk factors for AACG include an anteriorly positioned lens, short axial length, thick lens, and plateau iris configuration [10]. The risk of drug-induced AACG in space is increased as many of the anticholinesterase or cholinergic medications used by NASA to manage space motion sickness (such as scopolamine and promethazine) cause mydriasis which increases the risk of AACG, due to anterior displacement of lens-iris diaphragm [12]. With space tourism, individuals traveling to space may also be taking a variety of medications which are known as possible AACG precipitants, including α1-adrenergic agonists, β2 adrenergic agonists, sulfonamides, serotonergic agents, and antihistamines [10]. In addition to these risk factors, a dimly lit environment can also precipitate AACG, which may occur during spaceflight.
Ionizing radiation during long-duration spaceflight may cause cataracts, from exposure to galactic comic radiation or solar particle events. Radiation-induced cataract is a dose-dependent phenomena, and as the ionizing radiation dosage is increased, the lens becomes more opaque [13]. Local changes in the organized arrangement of lens cells caused by oxidation of protein sulfhydryl groups and the creation of high-molecular-weight complexes can lead to phacolytic glaucoma. Thus, cataract is a known risk factor for AACG. Although there have been no reported cases of astronauts developing AACG during or post-spaceflight, one additional rational for protective countermeasures against radiation exposure includes reduced risk for radiation-induced cataracts [14].
While the risks of AACG are rare with an estimated prevalence of 0.5–0.8% and have yet to be clearly defined in spaceflight, if AACG were to occur, the resulting visual loss and ocular pain could impact critical astronaut function and the mission [15]. Secondary optic nerve damage and visual loss if not rapidly treated aggressively and early can be permanent in AACG.
In the case of AACG in space, an immediate reduction in intra-ocular pressure via medical management would be the first step but on Earth depending on etiology a bilateral laser or surgical peripheral iridotomy may be necessary. As laser iridotomy is not available, medication to lower the IOP including topical ophthalmic beta blockers and muscarinic agonists is absolutely critical for future spaceflight.
Lower-body negative pressure (LBNP) is an emerging countermeasure for SANS by mitigating the cephalad fluid shifts in microgravity. However acute usage of LBNP in spaceflight did not impact choroidal thickness, which suggests that choroidal engorgement may occur secondary to cerebral venous congestion [16]. Gonioscopy is the gold standard method to examine the drainage angles and assess AACG risk, which can be performed quickly and with little patient discomfort. For objective measurement of the angle, ultrasound biomicroscopy or Visante OCT (Zeiss, Germany) can also be used for imaging. Further studies are required on the structural and functional effects on ocular structure on long-duration spaceflight [17,18,19].
Based on these considerations, we recommend that assessment of the angle be included in any ophthalmic screening prior to spaceflight in the era of civilian spaceflight and SANS. This includes a detailed medical history (including risk factors and current medications), optic nerve examination [20], and gonioscopy to identify for anatomical AACG risk factors. As we prepare for a future where spaceflight is more accessible to a civilian population with diverse physiology and anatomy, it becomes increasingly important to take into the considerations to reduce the risk of acute medical risks such as acute, sight-threatening glaucoma.
References
Stepanek J, Blue RS, Parazynski S (2019) Space medicine in the era of civilian spaceflight. Longo DL, ed. N Engl J Med 380(11):1053–1060. https://doi.org/10.1056/NEJMra1609012
Waisberg E, Ong J, Paladugu P et al (2022) Challenges of artificial intelligence in space medicine. Space: Sci Technol 2022:1–7. https://doi.org/10.34133/2022/9852872
Lee AG, Mader TH, Gibson CR et al (2020) Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. npj Micrograv 6(1):7. https://doi.org/10.1038/s41526-020-0097-9
Ong J, Tavakkoli A, Strangman G et al (2022) Neuro-ophthalmic imaging and visual assessment technology for spaceflight associated neuro-ocular syndrome (SANS). Surv Ophthalmol. Published online April 21, 2022:S0039–6257(22)00048–0. https://doi.org/10.1016/j.survophthal.2022.04.004
Patel ZS, Brunstetter TJ, Tarver WJ et al (2020) Red risks for a journey to the red planet: the highest priority human health risks for a mission to Mars. npj Micrograv 6(1):33. https://doi.org/10.1038/s41526-020-00124-6
Waisberg E, Ong J, Lee AG (2023) Factors associated with optic disc edema development during spaceflight. JAMA Ophthalmol. Published online March 16, 2023. https://doi.org/10.1001/jamaophthalmol.2023.0303
Waisberg E, Ong J, Masalkhi M, Lee AG (2023) Optic neuropathy in spaceflight-associated neuro-ocular syndrome (SANS). Ir J Med Sci. Published online April 1, 2023. https://doi.org/10.1007/s11845-023-03353-2
Iwasaki K ichi, Levine BD, Zhang R et al (2007) Human cerebral autoregulation before, during and after spaceflight: microgravity and dynamic cerebrovascular control. The J Physiol 579(3):799–810. https://doi.org/10.1113/jphysiol.2006.119636
Wilkerson MK, Muller-Delp J, Colleran PN, Delp MD (1999) Effects of hindlimb unloading on rat cerebral, splenic, and mesenteric resistance artery morphology. J Appl Physiol 87(6):2115–2121. https://doi.org/10.1152/jappl.1999.87.6.2115
Yang MC, Lin KY (2019) Drug-induced acute angle-closure glaucoma: a review. Journal of Current Glaucoma Practice 13(3):104–109. https://doi.org/10.5005/jp-journals-10078-1261
Yip JLY, Foster PJ (2006) Ethnic differences in primary angle-closure glaucoma. Curr Opin Ophthalmol 17(2):175–180. https://doi.org/10.1097/01.icu.0000193078.47616.aa
Brooks AMV, West RH, Gillies WE (1986) The risks of precipitating acute angle-closure glaucoma with the clinical use of mydriatic agents. Med J Aust 145(1):34–36. https://doi.org/10.5694/j.1326-5377.1986.tb113739.x
Lipman RM, Tripathi BJ, Tripathi RC (1988) Cataracts induced by microwave and ionizing radiation. Surv Ophthalmol 33(3):200–210. https://doi.org/10.1016/0039-6257(88)90088-4
Shah SS, Meyer JJ (2022) Lens induced glaucoma. In: StatPearls. StatPearls Publishing. Accessed December 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK574524/
Ramesh S, Maw C, Sutton CJ et al (2005) Ethnic aspects of acute primary angle closure in a UK mulicultural conurbation. Eye 19(12):1271–1275. https://doi.org/10.1038/sj.eye.6701759
Greenwald SH, Macias BR, Lee SMC et al (2021) Intraocular pressure and choroidal thickness respond differently to lower body negative pressure during spaceflight. J Appl Physiol 131(2):613–620. https://doi.org/10.1152/japplphysiol.01040.2020
Ong J, Tavakkoli A, Zaman N et al (2022) Terrestrial health applications of visual assessment technology and machine learning in spaceflight associated neuro-ocular syndrome. npj Microgravity 8(1):37. https://doi.org/10.1038/s41526-022-00222-7
Waisberg E, Ong J, Zaman N et al (2022) Head-mounted dynamic visual acuity for G-transition effects during interplanetary spaceflight: technology development and results from an early validation study. Aerosp med hum perform 93(11):800–805. https://doi.org/10.3357/AMHP.6092.2022
Ong J, Zaman N, Kamran SA et al (2022) A multi-modal visual assessment system for monitoring spaceflight associated neuro-ocular syndrome (SANS) during long duration spaceflight. J Vis 22(3):6. https://doi.org/10.1167/jov.22.3.6
Waisberg E, Micieli JA (2021) Neuro-ophthalmological optic nerve cupping: an overview. EB 13:255–268. https://doi.org/10.2147/EB.S272343
Funding
Open Access funding provided by the IReL Consortium.
Author information
Authors and Affiliations
Contributions
E.W.: conceptualization and writing. J.O.: conceptualization and writing. M.M.: conceptualization and writing. A.G.L.: review and intellectual support. J.B.: review and intellectual support.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Berdahl is the founder and CEO of Equinox Ophthalmic, Inc. The other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waisberg, E., Ong, J., Masalkhi, M. et al. Anatomical considerations for reducing ocular emergencies during spaceflight. Ir J Med Sci 193, 505–508 (2024). https://doi.org/10.1007/s11845-023-03407-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03407-5