Abstract
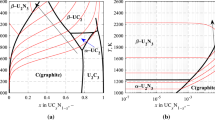
Carbides of uranium have attracted interest as fuels for nuclear thermal propulsion (NTP) to drive deep-space exploration owing to their attractive thermal and neutronic properties. Optimization of NTP technology, however, requires ultra-high temperature reactor environments to maximize the ratio of thrust to propellant to achieve peak rocket engine efficiency. Incorporation of transition metals into uranium carbides offers a pathway to increase the melting point of carbide fuels to address the operational challenges posed by NTP. A thermodynamic model has been developed to examine the phase relationships in the C–Ti–U system at NTP conditions. Calculated phase relationships at ultra-high temperatures predict a stable (U, Ti)C solid solution. Additionally, experimental work on C–Ti–U synthesis via arc melting has been carried out, providing data on phase constitution and compositions that can be used to further refine the thermodynamic model that has been developed.




Similar content being viewed by others
References
A. Accary, USAEC Report TID-7676 (1964)
W. Chubb and F.A. Rough, Battelle memorial institute report (BMI-1554) (1962)
H.S. Kalish, F.B. Litton, J. Crane, and M.L. Kohn, USAEC report NYO-2694 (1961)
H. Matzke, Science of Advanced LMFBR Fuels. (North-Holland, 1986)
C. Ganguly, P.V. Hegde, F. Jain, U. Basak, R. Mehrotra, and R. Roy, Nucl. Technol. 72(1), 59 (1986).
R.B. Matthews, and R.J. Herbst, Nucl. Technol. 63, 9 (1983).
I.L. Pioro, and R. Duffey, Current and future nuclear power reactors and plants. In Handbook of Generation IV Nuclear Reactors, ed. by I.L. Pioro IL. (Elsevier, Amsterdam, 2016)
C. Duguay, and G. Pelloquin, J. Eur. Ceram. Soc. 35(14), 3977 (2015).
F. Delage, J. Carmack, C.B. Lee, T. Mizuno, M. Pelletier, and J. Somers, J. Nucl. Mater. 441, 515 (2013).
Y. Orechwa, Y.I. Chang, M. Billone, and W.P. Barthold, Trans. Am. Nucl. Soc. 21, 406 (1975).
J. Zakova, and J. Wallenius, Ann. Nucl. Energy 47, 182 (2012).
S.K. Borowski, D.R. McCurdy, and T.W. Packard, in IEEE Aerospace Conference. (Big Sky, MT, 2012), pp. 1
B. Panda, R.R. Hickman, and S. Shah, NASA MSFC (2005)
W. Chubb, Nucl. Sci. Eng. 29, 176 (1967).
W. Chubb, BMI-1685, Batelle memorial institue (1964)
D.P. Butt, and T.C. Wallace, J. Am. Ceram. Soc. 76(6), 1109 (1993).
D.P. Butt, T.C. Wallace, W.A. Stark, E.K. Storms, F.G. Hampel, M.A. Williamson, and P.D. Kleinschmidt, LA-UR-92-1624. (Los Alamos National Laboratory, 1992)
D.G. Pelaccio, M.S. El-Genk, and D.P. Butt, AIP Conference Proceedings 301, 905 (1994)
Y. Katoh, G. Vasudevamurthy, T. Nozawa, and L.L. Snead (2020) Properties of zirconium carbide for nuclear fuel applications. In Comprehensive Nuclear Materials, ed. by R. Konings, and R. Stoller (Elsevier, Amsterdam), pp. 419
H. Holleck, J. Nucl. Mater. 124, 129 (1984).
H. Holleck, and H. Kleykamp, J. Nucl. Mater. 32(1), 1 (1969).
C.A. Millet, Commissariat à l'énergie atomique et aux énergies alternatives/CEA-R-3656 (1973)
F.A. Rough, and W. Chubb, Battelle memorial institute report (BMI-1441) (1960)
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics, The Calphad Method (Cambridge University Press, New York, 2007).
L. Kaufman, and H. Bernstein, Computer Calculations of Phase Diagrams (Academic, New York, 1970).
M. Perrut, Aerosp. Lab 9, 1 (2015).
G. Grimwall, Thermophysical Properties of Materials (Elsevier, Amsterdam, 1999).
C. Guéneau, N. Dupin, B. Sundman, C. Martial, J.-C. Dumas, S. Gossé, S. Chatain, F. De Bruycker, D. Manara, and R.J.M. Konings, J. Nucl. Mater. 419, 145 (2011).
A. Berche, N. Dupin, C. Guéneau, C. Rado, B. Sundman, and J.-C. Dumas, J. Nucl. Mater. 411(1–3), 131 (2011).
K. Frisk, Calphad 27, 367 (2003).
A.T. Dinsdale, Calphad 15(4), 317 (1991).
P.Y. Chevalier, and E. Fischer, J. Nucl. Mater. 288, 100 (2001).
R. Benz, C.G. Hoffman, and G.N. Rupert, High Temp. Sci. 1, 342 (1969).
E.K. Storms, and J. Griffin, High Temp. Sci. 5, 423 (1973).
A.E. Austin, Acta Crystallogr. 12, 159 (1959).
E.K. Storms, Los Alamos National Laboratory, LA-DC-972A (1968)
P.E. Potter, J. Nucl. Mater. 42, 1 (1972).
F. Robaut, et al., Microsc. Microanal. 12, 331 (2006).
M. Hillert, J. Alloys Compd. 320, 161 (2001).
Acknowledgements
The authors gratefully acknowledge the support of the U.S. Department of Energy through the LANL/LDRD Program and the G. T. Seaborg Institute for this work. The authors would also like to thank John Dunwoody for assistance with arc melting and Scarlett Widgeon Paisner for insightful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
As a supplement to this article, descriptions of the models used to calculate the liquid and solid-solution phases are described below.
Liquid Phase
The Two Sublattice Partially Ionic Liquid (TSPIL) model24 is used to model liquid phases as
where C, A, VA and B denote cation, anion, vacancy, and neutrally charged species, respectively. Charge neutrality necessitates that Q and P vary such that
vA and yA are the charge and site fractions of the anion species, and vC and yC are the charge and site fraction of the cation species C, respectively. The Gibbs energy of an ionic liquid can be expressed as
where \(^\circ G_{{{\text{C}}_{i} :{\text{A}}_{i} }}\) is the Gibbs energy of formation for vi + vj moles of atoms of the endmembers CiAj while \(^\circ G_{{{\text{C}}_{i} :{\text{A}}_{i} }}\) and \(^\circ G_{{{\text{C}}_{i} :{\text{A}}_{i} }}\) are the formation values for Ci and Bk.
Solid Solutions
The solid solutions were modeled using the compound energy formalism (CEF) was introduced by Hillert39 to describe the Gibbs energy of solid phases with sublattices. These phases have two or more sublattices, and at least one of these sublattices has a variable composition. Ideal entropy of mixing is assumed on each sublattice. This model is generally used to model crystalline solids, but it can also be extended to model ionic liquids.
Here, a solution phases with two sublattices, (A, B)a (C, D)b, will be used as an example to illustrate the compound energy formalism. In this model, components A and B can mix randomly on the first sublattice, as do the components C and D on the second sublattice. a and b are the corresponding stoichiometric coefficients. Site fraction \(y_{i}^{{\text{s}}}\) is introduced to describe the constitution of the phase and is defined as follows:
\(n_{i}^{{\text{s}}}\) is the number of component i on sublattice (s) and NS is the total number of sites on the same sublattice. When vacancies are considered in the model, the site fraction becomes
\(n_{{{\text{VA}}}}^{{\text{s}}} { }\) is the number of vacancies on sublattice (s). The site fraction can be transferred to mole fraction (xi) using the equation
When each sublattice is only occupied by one component, then end-members of the phase are produced. In the present case, four endmembers exist. They are AaCb, AaDb, BaCb, and BaDb. The surface of reference refGm is expressed as
The ideal entropy (idSm) and the excess free energy are expressed as
The binary interaction parameters Li,:k represent the interaction between the constituents i and j in the first sublattice when the second sublattice is only occupied by constituent k. These parameters can be further expanded with Redlich–Kister polynomial as
In the case of a three sublattice model:
Rights and permissions
About this article
Cite this article
Abdul-Jabbar, N.M., Ulrich, T.L. & White, J.T. Phase Relationships in the Carbon–Titanium–Uranium System for Ultra-High Temperature Nuclear Fuels. JOM 73, 3519–3527 (2021). https://doi.org/10.1007/s11837-021-04892-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-04892-8