Abstract
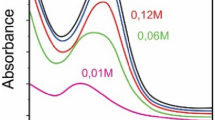
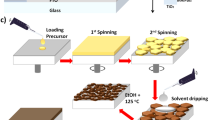
Antisolvent behavior is one of the most important treatments for producing high-quality perovskite MaPbI3 thin films. However, the optimization of the amount of antisolvent used has not been analyzed on a uniform platform. In this work, a systematic study is employed to quantitively evaluate the impact of anti-solvent treatment on the morphological, structural, and optoelectronic characteristics of MAPbI3 films. The results confirm that an adequate amount of 2.5 ml with a slow annealing treatment leads to homogeneous perovskite films with virtually no holes and large grain size. Using antisolvent treatment and optimized thermal annealing, we were able to control the nucleation and growth of the MAPbI3, and therefore achieve highly compact perovskite films with large grains, excellent crystalline quality, and very low pinhole density. The results of this study could help establish reproducible manufacturing processes for perovskite solar cells.






Similar content being viewed by others
References
A. Bouich, S. Ullah, B. Marí, L. Atourki, and M.E. Touhami, Mater. Chem. Phys. 258, 123973 (2021).
A. Bouich, S. Ullah, H. Ullah, M. Mollar, B. Marí, and M.E. Touhami, JOM 72(2), 615 (2020).
C. Quarti, E. Mosconi, J.M. Ball, V. D’Innocenzo, C. Tao, S. Pathak, and F.D. Angelis, Energy Environ. Sci. 9, 155 (2016).
J.H. Im, H.S. Kim, and N.G. Park, Appl. Mater. 2, 081510 (2014).
M.J. Carnie, C. Charbonneau, M.L. Davies, J. Troughton, T.M. Watson, K. Wojciechowski, and D.A. Worsley, Chem. Commun. 49, 7893 (2013).
D. Bi, S.J. Moon, L. Häggman, G. Boschloo, L. Yang, E.M. Johansson, and A. Hagfeldt, RSC Adv. 3, 18762 (2013).
Q. Chen, H. Zhou, Z. Hong, S. Luo, H.S. Duan, H.H. Wang, and Y. Yang, J. Am. Chem. Soc. 136, 622 (2013).
M.R. Leyden, L.K. Ono, S.R. Raga, Y. Kato, S. Wang, and Y. Qi, J. Mater. Chem. A 2, 18742 (2014).
W. Kong, Z. Ye, Z. Qi, B. Zhang, M. Wang, I.A. Rahimi, and H. Wu, Phys. Chem. Chem. Phys. 17, 16405 (2015).
X. Guo, C.M. Cleese, C. Kolodziej, A.C. Samia, Y. Zhao, and C. Burda, Dalton Trans. 45, 3806 (2016).
Z. Xiao, Q. Dong, C. Bi, Y. Shao, Y. Yuan, and J. Huang, Adv. Mater. 26, 6503 (2014).
S. Luo and W.A. Daoud, Materials 9, 123 (2016).
N.G. Park, CrystEngComm 18, 5977 (2016).
X. Zheng, B. Chen, C. Wu, and S. Priya, Nano Energy 17, 269 (2015).
Q. Jeangros, M. Duchamp, J. Werner, M. Kruth, R.E. Dunin-Borkowski, B. Niesen, and W.A. Hessler, Nano Lett. 16, 7013 (2016).
D. Liu, J. Yang, and T.L. Kelly, J. Am. Chem. Soc. 136, 17116 (2014).
H. Zhang, M. Lyu, Q. Wang, J.H. Yun, and L. Wang, Chem. Commun. 50, 11727 (2014).
J.J. Choi, X. Yang, Z.M. Norman, S.J. Billinge, and J.S. Owen, Nano Lett. 14, 127 (2013).
A. Halder, R. Chulliyil, A.S. Subbiah, T. Khan, S. Chattoraj, A. Chowdhury, and S.K. Sarkar, J. Phys. Chem. Lett. 6, 3483 (2015).
S. Ullah, A. Bouich, H. Ullah, B. Marí, and M. Mollar, Sol. Energy 208, 637 (2020).
A. Bouich, S. Ullah, H. Ullah, B. Marí, B. Hartiti, M.E. Touhami, and D.M.F. Santos, J. Mater. Sci.: Mater. 30(23), 20832 (2019).
G. Abdelmageed, L. Jewell, K. Hellier, L. Seymour, B. Luo, F. Bridges, and S. Carter, Phys. Lett. 109, 233905 (2016).
J. Li, Q. Dong, N. Li, and L. Wang, Adv. Energy Mater. 7, 1602922 (2017).
N. Aristidou, M.I. Sanchez, T. Chotchuangchutchaval, M. Brown, L. Martinez, T. Rath, and S.A. Haque, Angew. Chem. Int. Ed. 54, 8208 (2015).
Acknowledgement
This work was supported by Ministerio de Economia y Competitividad (Grant Number ENE2016-77798-C4-2-R).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bouich, A., Mari, B., Atourki, L. et al. Shedding Light on the Effect of Diethyl Ether Antisolvent on the Growth of (CH3NH3) PbI3 Thin Films. JOM 73, 551–557 (2021). https://doi.org/10.1007/s11837-020-04518-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-020-04518-5