Abstract
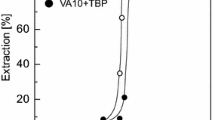
Extraction of scandium (Sc) from nickel processing residue using Cyanex 923 was studied. Cyanex 923 showed better selectivity for Sc compared with the other metallic components in the residue. Scandium was extracted by solvent extraction via a solvation mechanism. The aqueous-to-organic (A/O) phase ratio was set at 1:2. An increase in the scandium extraction rate was observed when increasing the concentration of H+. Cyanex 923 was shown to be more selective for Sc than Fe. Coextracted Fe was removed from the organic phase by 3 mol L−1 H2SO4 scrubbing with Sc loss of 1.3%. Stripping of scandium from the organic phase of 84.3% was achieved using 4% oxalic acid solution. The precipitated scandium oxalate was recovered by filtration and calcination. The obtained scandium oxide presented minimum purity of 99.0%.





Similar content being viewed by others
References
Z. Liu, H. Li, Q. Jing, and M. Zhang, JOM 69, 2373 (2017).
U.S. Geological Survey, Mineral Commodity Summaries 2015 (2015). https://minerals.usgs.gov/minerals/pubs/mcs/2015/mcs2015.pdf/. Accessed 31 Jan 2018.
U.S. Geological Survey, Mineral Commodity Summaries 2013 (2013). https://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf/. Accessed 31 Jan 2018.
J. Gambogi, Minerals Yearbook Rare Earths (2015). https://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/myb1-2014-raree.pdf/. Accessed 23 Nov 2017.
B. Onghena, C.R. Borra, T.V. Gerven, and K. Binnemans, Sep. Purif. Technol. 176, 208 (2017).
S. Wang, JOM 65, 1317 (2013).
L.T. Peiró and G.V. Méndez, JOM 65, 1327 (2013).
F. Xie, T.A. Zhang, D. Dreisinger, and F. Doyle, Miner. Eng. 56, 10 (2014).
W. Wang, Y. Pranolo, and C. Cheng, Metall. Sci Technol. 25, 11 (2007).
G. Gongyi, C. Yuli, and L. Yu, JOM 40, 28 (1988).
B.R. Reddy, D.N. Priya, S.V. Rao, and P. Radhika, Hydrometallurgy 77, 253 (2005).
B.R. Reddy and D.N. Priya, J. Power Sources 161, 1428 (2006).
Cytec, Cyanex 272 extractant (Cytec Industries Inc., 2008). https://www.cytec.com/sites/default/files/datasheets/CYANEX%20272%20Brochure.pdf. Accessed 20 July 2018.
K. Sarangi, P.K. Parhi, E. Padhan, A.K. Palai, K.C. Nathsarma, and K.H. Park, Sep. Purif. Technol. 55, 44 (2007).
M. Haslam and B. Arnall, ALTA 1999—Nickel/Cobalt Pressure Leaching & Hydrometallurgy Forum (Perth, 1999).
C. Wang and D. Li, Solvent Extr. Ion Exchange 12, 615 (1994).
L. Bykhovsky and L. Tigunov, International Geological Congress (Oslo, 2008).
D. Li and C. Wang, Hydrometallurgy 48, 301 (1998).
Cytec, Cyanex 923 extractant (Cytec Industries Inc., 2008). https://www.cytec.com/sites/default/files/datasheets/SPT-032-D.pdf. Accessed 23 April 2018.
V. Ribeiro and R. Santos, Breve Revisão Bibliográfica dos Processos de Lixiviação de Minérios e Concentrados de Terras-Raras (Rio de Janeiro: CETEM/MCTI, 2014), pp. 14–15.
B. Gupta, A. Deep, P. Malik, and S.N. Tandon, Solvent Extr. Ion Exchange 20, 81 (2002).
G. Ritcey, Solvent Extraction: Principles and Applications to Process Metallurgy (Ottawa: Elsevier, 2006), pp. 51–75.
V. Kislik, Solvent Extraction: Classical and Novel Approaches, 1st ed. (Amsterdam: Elsevier, 2012), pp. 323–326.
R. Vickery, J. Chem. Soc. 0, 3113 (1956).
Acknowledgements
Financial support for this study was provided by the São Paulo Research Foundation (FAPESP—Grant 2012/51871-9). This study was partly financed by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES) Finance Code 001. The authors thank Ana Carolina Fadel Dalsin for her help on the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souza, A.G.O., Aliprandini, P., Espinosa, D.C.R. et al. Scandium Extraction from Nickel Processing Waste Using Cyanex 923 in Sulfuric Medium. JOM 71, 2003–2009 (2019). https://doi.org/10.1007/s11837-019-03427-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03427-6