Abstract
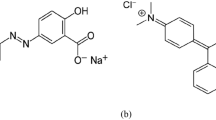
Sewage sludge biochar (SBC) was used as adsorbent to study the adsorption behavior of triarylmethane dyes, malachite green (MG; diaminotriphenylmethane), and crystal violet (CV; triaminotriphenylmethane). SBC exhibited high content (g/kg) of Al (65.8), P (64.6), Ca (57.3), and Fe (44.6). The Langmuir model showed that the affinity of MG for the surface of SBC was 22.6-times that of CV’s (KL=0.0053l/mg); maximum Langmuir monolayer adsorption capacity of 69.5 mg/g for MG and 49.0 mg/g for CV. Similar functional groups and adsorption mechanisms like hydrogen bonding, π-π interaction, electrostatic interactions, and ion exchanges governed both MG and CV adsorption onto SBC. Both physisorption and chemisorption were involved in both dyes’ adsorption (Redlich-Peterson model: R2> 0.900) Leachability tests showed a dependency of leached metallic ions on the type of dye employed, where ion exchange was dominated by P, Al, Ca, K for MG, and Na, K, Ca for CV. Interestingly, although minimal, the standalone contribution of biochar-free ions on MG and CV decolorization was, respectively, 13% and 7.7% (Fe), 6.7% and 2.3% (K), 2.9% and 0% (Ca), and 0% and 0.8% (Mg), which showed that some adsorption-unrelated mechanism may have also contributed to decolorization of CV and MG.
Similar content being viewed by others
References
V. Katheresan, J. Kansedo and S. Y. Lau, J. Environ. Chem. Eng., 6, 4676 (2018).
M. T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Adv. Colloid Interface Sci., 209, 172 (2014).
T. Robinson, G. McMullan, R. Marchant and P. Nigam, Bioresour. Technol., 77, 247 (2001).
C. J. Ogugbue and T. Sawidis, Biotechnol. Res. Int., 2011, 1 (2011).
V. Gupta, I. Ali and D. Mohan, J. Colloid Interface Sci., 265, 257 (2003).
J. Liu, Z. Wang, H. Li, C. Hu, P. Raymer and Q. Huang, Bioresour. Technol., 249, 307 (2018).
P. S. Kumar, S. J. Varjani and S. Suganya, Bioresour. Technol., 250, 716 (2018).
N. M. Mahmoodi and M. H. Saffar-Dastgerdi, Microchem. J., 145, 74 (2019).
G. Ohemeng-Boahen, D. D. Sewu and S. H. Woo, Environ. Sci. Pollut. Res., 26, 33030 (2019).
D. D. Sewu, P. Boakye, H. Jung and S. H. Woo, Bioresour. Technol., 244, 1142 (2017).
D. Mohan, A. Sarswat, Y. S. Ok and C. U. Pittman Jr., Bioresour. Technol., 160, 191 (2014).
D. D. Sewu, H. Jung, S. S. Kim, D. S. Lee and S. H. Woo, Bioresour. Technol., 277, 77 (2019).
P. Boakye, H. N. Tran, D. S. Lee and S. H. Woo, J. Environ. Manage., 233, 165 (2019).
D. D. Sewu, D. S. Lee, H. N. Tran and S. H. Woo, J. Taiwan Inst. Chem. E., 104, 106 (2019).
H.-O. Chahinez, O. Abdelkader, Y. Leila and H. N. Tran, Environ. Technol. Inno., 19, 100872 (2020).
D. Kalderis, B. Kayan, S. Akay, E. Kulaksız and B. Gözmen, J. Environ. Chem. Eng., 5, 2222 (2017).
K. Smith, G. Fowler, S. Pullket and N. J. D. Graham, Water Res., 43, 2569 (2009).
B. Xiao, Q. Dai, X. Yu, P. Yu, S. Zhai, R. Liu, X. Guo and H. Chen, J. Hazard. Mater., 343, 347 (2018).
P. Oleszczuk, S. E. Hale, J. Lehmann and G. Cornelissen, Bioresour. Technol., 111, 84 (2012).
N. C. Shiba and F. Ntuli, Waste Manage., 60, 191 (2017).
K. Phoungthong, H. Zhang, L.-M. Shao and P.-J. He, J. Mater. Cycles Waste Manage., 20, 2089 (2018).
L. Leng, X. Yuan, H. Huang, J. Shao, H. Wang, X. Chen and G. Zeng, Appl. Surf. Sci., 346, 223 (2015).
D. D. Sewu, P. Boakye and S. H. Woo, Bioresour. Technol., 224, 206 (2017).
E. Commission, Offic. J. Eur. Comm., 181, 6 (1986).
G. Newcombe, R. Hayes and M. Drikas, Colloids Surf. A, 78, 65 (1993).
I. Langmuir, J. Am. Chem. Soc., 38, 2221 (1916).
L. Yao, J. Yang, P. Zhang and L. Deng, Bioresour. Technol., 256, 208 (2018).
H. N. Tran, S.-J. You, A. Hosseini-Bandegharaei and H.-P. Chao, Water Res., 120, 88 (2017).
M. Dubinin, Chem. Rev., 60, 235 (1960).
T. W. Weber and R. K. Chakravorti, AIChE J., 20, 228 (1974).
S. K. Low and M. C. Tan, J. Environ. Chem. Eng., 6, 3502 (2018).
S. Lagergren, SvenVetenskapsakad. Handingarl, 24, 1 (1898).
G. Blanchard, M. Maunaye and G. Martin, Water Res., 18, 1501 (1984).
Y S. Ho, D. J. Wase and C. Forster, Environ. Technol., 17, 71 (1996).
W. J. Weber and J. C. Morris, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 89, 31 (1963).
G. Boyd, A. Adamson and L. Myers Jr., J. Am. Chem. Soc., 69, 2836 (1947).
D. Reichenberg, J. Am. Chem. Soc., 75, 589 (1953).
G. F. Malash and M. I. El-Khaiary, Chem. Eng. J., 163, 256 (2010).
C. Yao and T. Chen, Chem. Eng. Res. Des., 119, 87 (2017).
I. Tan, B. Hameed and A. Ahmad, Chem. Eng. J., 127, 111 (2007).
S. Schimmelpfennig and B. Glaser, J. Environ. Qual., 41, 1001 (2012).
O. Krüger, A. Grabner and C. Adam, Environ. Sci. Technol., 48, 11811 (2014).
J. Coates, in Encyclopedia of analytical chemistry: Applications, Theory and instrumentation, R. A. Meyers Ed., John Wiley and Sons Ltd, Chichester (2000).
X. Zhao, X. Bu, T. Wu, S.-T. Zheng, L. Wang and P. Feng, Nat. Commun., 4, 1 (2013).
R. Tabaraki and N. Sadeghinejad, Water Sci. Technol., 75, 2631 (2017).
H. N. Tran, S.-J. You and H.-P. Chao, Korean J. Chem. Eng., 34, 1708 (2017).
Y. Ho and G. McKay, Can. J. Chem. Eng., 76, 822 (1998).
E. Castellini, R. Andreoli, G. Malavasi and A. Pedone, Colloids Surf. A., 329, 31 (2008).
A. R. Abbasi, M. Karimi and K. Daasbjerg, Ultrason. Sonochem., 37, 182 (2017).
A. Wathukarage, I. Herath, M. Iqbal and M. Vithanage, Environ. Geochem. Health, 41, 1647 (2019).
X. Chen, L. Yang, S. C. Myneni and Y. Deng, Chem. Eng. J., 373, 840 (2019).
K. Singh and S. Arora, Crit. Rev. Environ. Sci. Technol., 41, 807 (2011).
T. Santhi, S. Manonmani and T. Smitha, J. Hazard. Mater., 179, 178 (2010).
L. Leng, X. Yuan, G. Zeng, J. Shao, X. Chen, Z. Wu, H. Wang and X. Peng, Fuel, 155, 77 (2015).
M. Yu, Y. Han, J. Li and L. Wang, Chem. Eng. J., 317, 493 (2017).
E. Akar, A. Altinişik and Y. Seki, Ecol. Eng., 52, 19 (2013).
M. Choudhary, R. Kumar and S. Neogi, J. Hazard. Mater., 392, 122441 (2020).
S.-S. Yang, J.-H. Kang, T.-R. Xie, L. He, D.-F. Xing, N.-Q. Ren, S.-H. Ho and W.-M. Wu, J. Cleaner Prod., 227, 33 (2019).
S. Madhavakrishnan, K. Manickavasagam, R. Vasanthakumar, K. Rasappan, R. Mohanraj and S. Pattabhi, E-J. Chem., 6, 1109 (2009).
F. Güzel, H. Sayğılı, G. A. Sayğılı and F. Koyuncu, J. Ind. Eng. Chem., 20, 3375 (2014).
H. Jung, D. D. Sewu, G. Ohemeng-Boahen, D. S. Lee and S. H. Woo, Waste Manage., 91, 33 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
Additional information as noted in the text. This information is available via the Internet at u]http://www.springer.com/chemistry/journal/11814.
Supporting Information
11814_2020_727_MOESM1_ESM.pdf
Decolorization of triarylmethane dyes, malachite green, and crystal violet, by sewage sludge biochar: Isotherm, kinetics, and adsorption mechanism comparison
Rights and permissions
About this article
Cite this article
Sewu, D.D., Lee, D.S., Woo, S.H. et al. Decolorization of triarylmethane dyes, malachite green, and crystal violet, by sewage sludge biochar: Isotherm, kinetics, and adsorption mechanism comparison. Korean J. Chem. Eng. 38, 531–539 (2021). https://doi.org/10.1007/s11814-020-0727-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-020-0727-7