Abstract
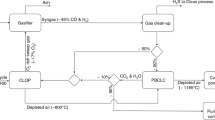
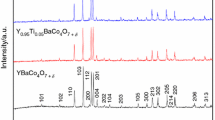
Chemical looping air separation gives an energy-efficient choice for oxygen production. We performed kinetic analysis of YBaCo4O7+δ, Y0.95Ti0.05BaCo4O7+δ, Y0.2Ti0.05Dy0.75BaCo4O7+δ, and Y0.15Zr0.1Dy0.75BaCo4O7+δ oxygen carriers in a CLAS process. TG experiments were conducted with heating rates of 0.5, 1, and 2 °C/min in a thermogravimetric analyzer. Further exploration is required to develop an appropriate oxygen carrier. So, we used the model-free approach, Starink method, to evaluate the apparent activation energy. And, masterplots method was applied to determine the most probable mechanism function. The results show that the distributed activation energies of oxidation/reduction process are 189.42/286.22 kJ/mol, 197.70/324.87 kJ/mol, 195.41/310.4 kJ/mol, and 192.20/293.53 kJ/mol for YBaCo4O7+δ, Y0.95Ti0.05BaCo4O7+δ, Y0.2Ti0.05Dy0.75BaCo4O7+δ, and Y0.15Zr0.1Dy0.75BaCo4O7+δ oxygen carriers, respectively. Random nucleation and nuclei growth A model is the most suitable for oxidation process. The A model and D are the most suitable for the reduction process. Regarding YBaCo4O7+δ, Y0.95Ti0.05BaCo4O7+δ, Y0.2Ti0.05Dy0.75BaCo4O7+δ, and Y0.15Zr0.1Dy0.75BaCo4O7+δ kinetic, oxygen transfer materials are rate-determined by nucleation and nuclei growth. For reduction kinetic, the gas diffusion stage could also become a dominant step.
Similar content being viewed by others
References
K. Shah, B. Moghtaderi, J. Zanganeh and T. Wall, Fuel, 107, 356 (2013).
A.R. Smith and J. Klosek, Fuel Process Technol., 70(2), 115 (2001).
B. Moghtaderi, Energy Fuels, 24(1), 190 (2010).
H. Song, K. Shah, E. Doroodchi, T. Wall and B. Moghtaderi, Energy Fuel, 28, 173 (2013).
H. Song, K. Shah, E. Doroodchi and B. Moghtaderi, Energy Fuel, 28, 163 (2014).
A. Shulman, E. Cleverstam, T. Mattisson and A. Lyngfelt, Energy Fuel, 23, 5269 (2009).
K. Wang, Q. B. Yu, H.Q. Xie and Q. Qin, Funct. Mater. Lett., 6(2), 1350022(1) (2013).
H. Song, K. Shah, E. Doroodchi and B. Moghtaderi, Energy Fuel, 28, 1284 (2014).
K. Wang, Q. B. Yu, Q. Qin and Z. L. Zuo, J. Therm. Anal. Calorim., 119, 2221 (2014).
K. Wang, Q.B. Yu and Q. Qin, Energy Fuel, 27(9), 5466 (2013).
M. Ishida, M. Yamamoto and T. Ohba, Energy Convers and Manag., 43, 1469 (2002).
T. Mattisson, H. Leion and A. Lyngfelt, Fuel, 88, 683 (2009).
M. Arjmand, A. Azad, H. Leion, A. Lyngfelt and T. Mattisson, Energy Fuel, 25(11), 5493 (2011).
K. Wang, Q. B. Yu and Q. Qin, J. Therm. Anal. Calorim., 112(2), 747 (2013).
G. Azimi, H. Leion, M. Rydén, T. Mattisson and A. Lyngfelt, Energy Fuel, 27(1), 367 (2013).
K. Wang, Q. B. Yu, Q. Qin and Z. Zuo, J. Therm. Anal. Calorim., 119(3), 2221 (2015).
K. Zhao, F. He, Z. Huang, G.Q. Wei, A.Q. Zheng, H.B. Li and Z. L. Zhao, Korean J. Chem. Eng., 34(6), 1651 (2017).
B.Y. Kwak, N. K. Park, J. I. Baek, H. J. Ryu and M. Kang, Korean J. Chem. Eng., 34(7), 1936 (2017).
T. Motohashi, S. Kadita, H. Fjellvag, M. Karppinen and H. Yamauchi, Mater. Sci. Eng. B., 148(1), 196 (2008).
M. Karppinen, H. Yanauchi, S. Otani, T. Fujita, T. Motohashi, Y.-H. Huang, M. Valkeapää and H. Fjellvåg,, Chem. Mater., 18(2), 490 (2006).
S. Kadita, M. Kappinen, T. Motohashi and H. Yamauchi, Chem. Mater., 20, 6378 (2008).
S. Wang, H. S. Hao, B. F. Zhu, J. F. Jia and X. Hu, J. Mater. Sci., 43, 5385 (2008).
H. S. Hao, Q. L. He, Y.G. Cheng and L.M. Zhao, J. Phys. Chem. Solids., 75(4), 495 (2014).
S.M. Zhang, MA Dissertation, ZhengZhou University (2011).
L. J. Guo, MA Dissertation, ZhengZhou University (2005).
L. P. Kozeeva, M.Y. Kameneva, A.N. Lavrov and N.V. Podberezskaya, Inorg Mater., 49(6), 626 (2013).
O. Parkkima, H. Yamauchi and M. Karppinen, Chem. Mater., 25(4), 599 (2013).
V. Martin, Solid State Sci., 7(10), 1163 (2005).
S. Räsänen, T. Motohashi, H. Yamauchi and M. Kappinen, J. Solid State Chem., 183, 692 (2010).
T. Komiyama, T. Motohashi, Y. Masubuchi and S. Kikkawa, Mater. Res. Bull., 45, 1527 (2010).
R. Samuli, P. Outi, R. Eeva-Leena, Y. Hisao and K. Maarit, Solid State Ionics., 208, 31 (2012).
B. Jankovic, B. Adnadevic and J. Jovanovic, Thermochim. Acta, 452, 106 (2007).
S. Vyazovkin, Thermochim. Acta, 355, 145 (2000).
M. E. Brown, D. Dollimore and A. K. Galwey, Elsevier, Amsterdam., 22, 41 (1980).
S. Vyazovkin and C.A. Wight, Thermachim. Acta, 341, 53 (1999).
S. Vyazovkin and C. A. Wight, J. Phys. Chem. A., 101(39), 7217 (1997).
A.W. Coats and J. P. Redfern, Nature, 201, 68 (1964).
A.W. Coats and J. P. Redfern, J. Polym. Sci. Part B: Polym. Lett., 3, 917 (1965).
T. Ozawa, Bull. Chem. Soc. Japan, 38, 1881 (1965).
C.D. Doyle, Anal. Chem., 33, 77 (1961).
C.D. Doyle, J. Appl. Polym. Sci., 5, 285 (1961).
C.D. Doyle, Nature, 207, 290 (1965).
H. E. Kissinger, Anal. Chem., 29, 1702 (1957).
T. Akahira and T. Sunose, Res. Rep. Chiba. Inst. Technol., 16, 22 (1971).
S.V. Vyazovkin and A. I. Lesnikovich, Thermochim. Acta, 34(3), 609 (1988).
P.K. Agrawal, Thermochim. Acta, 203, 93 (1992).
M. J. Starink, Thermochim. Acta, 288, 97 (1996).
S. Vyazovkina, A.K. Burnhamb, J. M. Criadoc, A. L. Pérez-Maquedac, C. Popescud and N. Sbirrazzuolie, Thermochim. Acta, 520(1-2), 1 (2011).
T. Wanjun, L. Yuwen, Z. Hen and W. Cunxin, Thermochim. Acta, 74, 309 (2003).
F. J. Gotor, J. M. Criado, J. Malek and M. Koga, J. Phys. Chem. A., 104, 10777 (2000).
T. Wanjun, L. Yuwen, Z. Hen and W. Cunxin, Thermochim. Acta, 74, 309 (2003).
H.G. Jin, T. Okamoto and M. Ishida, Energy Fuel, 12, 1272 (1998).
I. Halikia, P. Neou-Syngouna and D. Kolitsa, Thermochim. Acta, 320(1–2), 75 (1998).
C. Perkins, P. Lichty and A.W. Weimer, Chem. Eng. Sci., 62(21), 5952 (2007).
A. Pineau, N. Kanari and I. Gaballah, Thermochim. Acta, 447(1), 89 (2006).
M.M. Hossain and H. I. de Lasa, Chem. Eng. Sci., 65, 98 (2010).
M.M. Hossain and H. I. de Lasa, Chem. Eng. Sci., 63, 4433 (2008).
Y.Q. Sun, S. Sridhar, S. Seetharaman, H. Wang, L. L. Liu, X.D. Wang and Z.T. Zhang, Sci. Rep., 6, 1 (2016), DOI:10.1038/srep22323.
M.M. Hossain and H. I. de Lasa, Chem. Eng. Sci., 65, 98 (2010).
M.M. Hossain and H. I. de Lasa, Chem. Eng. Sci., 63, 4433 (2008).
J.D. Hancock and J. H. Sharp, J. Am. Ceram. Soc., 55(2), 74 (1972).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, L., Yu, Q., Wang, T. et al. Kinetics of perovskite-like oxygen carriers for chemical looping air separation. Korean J. Chem. Eng. 35, 626–636 (2018). https://doi.org/10.1007/s11814-017-0332-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-017-0332-6