Abstract
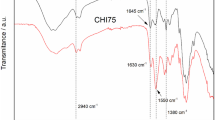
Uniform molecular weight (Mw) chitosan (CS) is highly demanded in medical biomaterial industry. This present article described heterogeneous degradation of CS in aqueous HCl/ethanol solution, in which progress uniform Mw CS was successfully prepared. The Mw distribution of CS was measured by gel permeation chromatography (GPC) analysis. Moreover, the structure and properties of degraded CS were characterized by Fourier transform infrared spectroscopy (FT-IR), nuclear magnetic resonance spectroscopy (1H NMR), X-ray diffraction (XRD) and thermogravimetric (TG) analysis. In addition, the biocompatibility of degraded CS was also assessed by hemolysis rate (HR) measurement. The Mw of CS dramatically decreased from 246 KDa to 76 kDa at the initial 30min, and stabilized at 18 kDa after 24 h. GPC analysis results showed that the degraded CS molecular become homogenization. FT-IR and ’H NMR analysis confirmed the basic structure of CS molecular backbone was not destroyed during this progress. Besides, the water solubility of CS was not significantly influenced by this reaction. Moreover, the XRD analysis revealed that crystallinity of degraded CS increased from 70.32% to 99.25% with time. The TG analysis showed improved thermal stability of degraded CS. HR measurement demonstrated the degraded CS possessed excellent biocompatibility. This simple and efficient heterogeneous degradation would open up a new route to produce uniform Mw CS.
Similar content being viewed by others
References
Cabrera, J. C, and Van Cutsem, P., 2005. Preparation of chitoo-ligosaccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochemical Engineering Journal, 25 (2): 165–172.
Chan, L. W, Kim, C. H., Wang, X., Pun, S. H., White, N. I, and Kim, T. H., 2016. PolySTAT-modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta Biomate-rialia, 31: 178–185.
Chen, Q., Xiao, W., Zhou, L., Wu, T., and Wu, Y, 2012. Hydrolysis of chitosan under microwave irradiation in ionic liq- uids promoted by sulfonic acid-functionalized ionic liquids. Polymer Degradation and Stability, 97 (1): 49–53.
Cheung, R., Ng, T., Wong, J., and Chan, W., 2015. Chitosan: An update on potential biomedical and pharmaceutical applications. Marine Drugs, 13 (8): 5156–5186.
Chokradjaroen, C, Rujiravanit, R., Watthanaphanit, A., Theera-munkong, S., Saito, N., Yamashita, K, and Arakawa, R., 2017. Enhanced degradation of chitosan by applying plasma treatment in combination with oxidizing agents for potential use as an anticancer agent. Carbohydrate Polymers, 167: 1–11.
Cocarta, A. I., Gutanu, V, and Dragan, E. S., 2017. Structural, morphological and magnetic characterization of metal-chito-san/poly (vinyl amine) complexes. Journal of Polymer Research, 24 (2): 20.
Corazzari, I., Nistico, R., Turci, E, Faga, M. G, Franzoso, F., Tabasso, S., and Magnacca, G, 2015. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polymer Degradation and Stability, 112: 1–9.
Dorati, R., Pisani, S., Maffeis, G, Conti, B., Modena, T., Chiesa, E., and Genta, I., 2018. Study on hydrophilicity and degrad-ability of chitosan/polylactide-co-polycaprolactone nanofibre blend electrospun membrane. Carbohydrate Polymers, 199: 150–160.
Furuike, T., Komoto, D., Hashimoto, H, and Tamura, H, 2017. Preparation of chitosan hydrogel and its solubility in organic acids. International Journal of Biological Macromolecules, 104: 1620–1625.
Gao, T. T., Kong, M., Cheng, X. J., Xia, G X., Gao, Y Y, Chen, X. G, and Park, H. J., 2014. A thermosensitive chitosan-based hydrogel for controlled release of insulin. Frontiers of’Materials Science, 8 (2): 142–149.
Hamedi, H, Moradi, S., Hudson, S. M., and Tonelli, A. E., 2018. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydrate Polymers, 199: 445–460.
Huang, Q. Z., Wang, S. M., Huang, J. E, Zhuo, L. H, and Guo, Y C, 2007. Study on the heterogeneous degradation of chitosan with hydrogen peroxide under the catalysis of phos-photungstic acid. Carbohydrate Polymers, 68 (4): 761–765.
Huang, Q. Z., Zhuo, L. H, and Guo, Y C, 2008. Heterogeneous degradation of chitosan with H2O2 catalysed by phosphotung-state. Carbohydrate Polymers, 72 (3): 500–505.
Kalita, N. K, Nagar, M. K, Mudenur, C, Kalamdhad, A., and Katiyar, V, 2019. Biodegradation of modified Poly (lactic acid) based biocomposite films under thermophilic composting conditions. Polymer Testing, 76: 522–536.
Kloster, G A., Muraca, D., Londono, O. M., Knobel, M., Mar-covich, N. E., and Mosiewicki, M. A., 2018. Structural analysis of magnetic nanocomposites based on chitosan. Polymer Testing, 72: 202–213.
Li, I, Ma, F K, Dang, Q. E, Liang, X. G, and Chen, X. G, 2014. Glucose-conjugated chitosan nanoparticles for targeted drug delivery and their specific interaction with tumor cells. Frontiers of Materials Science, 8 (4): 363–372.
Li, K, Xing, R., Liu, S., Qin, Y, Meng, X., and Li, P., 2012. Microwave-assisted degradation of chitosan for a possible use in inhibiting crop pathogenic fungi. International Journal of Biological Macromolecules, 51 (5): 767–773.
Li, X., Tang, J., Bao, L., Chen, L., and Hong, E E, 2017. Performance improvements of the BNC tubes from unique dou-ble-silicone-tube bioreactors by introducing chitosan and heparin for application as small-diameter artificial blood ves- sels. Carbohydrate Polymers, 178: 394–405.
Li, Y, Li, J., Liu, T., Wang, Y, Zhou, Z., Cheng, R, and Chen, X., 2017. Preparation and antithrombotic activity identification of Perinereis aibuhitensis extract: A high temperature and wide pH range stable biological agent. Food and Function, 8 (10): 3533–3541.
Liang, D., Lu, Z., Yang, H, Gao, J., and Chen, R., 2016. Novel asymmetric wettable AgNPs/chitosan wound dressing: In vitro and in vivo evaluation. ACS Applied Materials and Interfaces, 8 (6): 3958–3968.
Lim, L. M., Tran, T. L, Wong, J. J. L., Wang, D., Cheow, W. S., and Hadinoto, K., 2018. Amorphous ternary nanoparticle complex of curcumin-chitosan-hypromellose exhibiting built-in solubility enhancement and physical stability of curcumin. Colloids and Surfaces B: Biointerfaces, 167: 483–491.
Liu, N., Chen, X. G, Park, H. J., Liu, C. G, Liu, C. S., Meng, X. H., and Yu, L. J., 2006. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydrate Polymers, 64 (1): 60–65.
Nguyen, A. T., Huang, Q. L., Yang, Z., Lin, N, Xu, G, and Liu, X. Y, 2015. Crystal networks in silk fibrous materials: From hierarchical structure to ultra performance. Small, 11 (9-10): 1039–1054.
Nidheesh, L, Kumar, P. G, and Suresh, P. V, 2015. Enzymatic degradation of chitosan and production of d-glucosamine by solid substrate fermentation of exo-P-d-glucosaminidase (exochitosanase) by Penicillium decumbens CFRNT15. International Biodeterioration and Biodegradation, 97: 97–106.
Osorio-Madrazo, A., David, L., Trombotto, S., Lucas, J. M., Peniche-Covas, C, and Domard, A., 2010. Kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromole-cules, 11(5): 1376–1386.
Shen, S. S., Yang, J. J., Liu, C. X., and Bai, R. B., 2017. Immobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption. RSC Advances, 7(17): 10424–10431.
Sun, G, Zhang, X., Bao, Z., Lang, X., Zhou, Z., Li, Y, and Chen, X., 2018. Reinforcement of thermoplastic chitosan hy-drogel using chitin whiskers optimized with response surface methodology. Carbohydrate Polymers, 189: 280–288.
Varum, K. M., Ottey, M. H., and Smidsred, O., 2001. Acid hy- drolysis of chitosans. Carbohydrate Polymers, 46 (1): 89–98.
Wang, D., Zhao, L, Yan, L., Mi, Z., Gu, Q., and Zhang, Y, 2016. Synthesis, characterization and evaluation of dewatering properties of chitosan-grafting DMDAAC llocculants. International Journal of Biological Macromolecules, 92: 761–768.
Wang, S. M., Huang, Q. Z., and Wang, Q. S., 2005. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydrate Research, 340 (6): 1143–1147.
Wu, J., Guo, L., Wen, M., Bu, L, Zhou, P., Zhong, J., and Zhang, Q., 2019. The self-assembling growth of copper nanowires for transparent electrodes. Journal of Wuhan University of Technology-Materials Science Edition, 34 (1): 145–149.
Wu, T., Wu, C, Xiang, Y, Huang, J., Luan, L., Chen, S., and Hu, Y, 2016. Kinetics and mechanism of degradation of chitosan by combining sonolysis with H2O2/ascorbic acid. RSC Advances, 6 (80): 76280–76287.
Xia, R., Cao, X., Gao, M., Zhang, P., Zeng, M., Wang, B., and Wei, L., 2017. Probing sub-nano level molecular packing and correlated positron annihilation characteristics of ionic cross-linked chitosan membranes using positron annihilation spectroscopy. Physical Chemistry Chemical Physics, 19 (5): 3616–3626.
Younes, I., and Rinaudo, M., 2015. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs, 13(3): 1133–1174.
Yu, X., Mu, Y, Xu, M., Xia, G, Wang, J., Liu, Y, and Chen, X., 2017. Preparation and characterization of mucosal adhesive and two-step drug releasing cetirizine-chitosan nanoparticle. Carbohydrate Polymers, 173: 600–609.
Zhang, H, and Neau, S. H, 2002. In vitro degradation of chitosan by bacterial enzymes from rat cecal and colonic contents. Biomaterials, 23 (13): 2761–2766.
Zhou, H. Y, Zhou, D. J., Zhang, W. E, Jiang, L. J., Li, J. B., and Chen, X. G, 2011. Biocompatibility and characteristics of chitosan/cellulose acetate microspheres for drug delivery. Frontiers of Materials Science, 5 (4): 367–378.
Zou, P., Yang, X., Wang, J., Li, Y, Yu, H, Zhang, Y, and Liu, G, 2016. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chemistry, 190: 1174–1181.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. U1706212 and 81671828).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, X., Li, Y., Sun, G. et al. Researches on the Internal Molecular Weight Uniformity of Chitosan Biomaterials. J. Ocean Univ. China 19, 459–465 (2020). https://doi.org/10.1007/s11802-020-4192-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-020-4192-z