Abstract
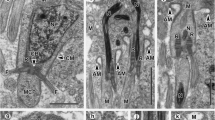
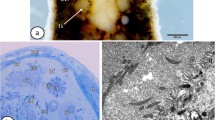
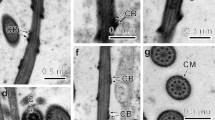
Spionid worms of Polydora ciliata inhabit the shells of many commercially important bivalves and cause disease in molluscan aquaculture. Their sperm structure is closely related to their fertilization method. To give an insight into the sperm structure and spermatogenesis, ultrastructure details of the subcellular components of germ cells during spermiogenesis of Polydora ciliata are detected by transmission electron microscopy (TEM). In P. ciliata, during spermiogenesis, chromatin is regularly arranged as dense fibrils and becomes more condensed when the nucleus elongates. Microtubules do not surround the nucleus during its elongation. The Golgi phase is characterized by the formation of proacrosomal granules within the Golgi apparatus. The proacrosomal granules fuse to form a single, spherical acrosomal vesicle that migrates to the anterior pole of the cell. At the time of nuclear condensation, mitochondria become reduced in number but increased in size, causing deep indentation at the base of the nucleus. The mid-piece has a few mitochondria. The cap phase includes the spreading of the acrosomal granule over the surface of the nucleus of the differentiating spermatid. The acrosomal phase of spermiogenesis is typically associated with changes in the shape of the nucleus, acrosome and tail. The relationship of sperm ultrastructure to spermiogenesis in spionidae species was discussed.
Similar content being viewed by others
References
Aviles, F., Rozbaczylo, N., Herve, M., and Godoy, M., 2007. First report of Polychaetes from the genus Oriopsis (Polychaeta: Sabellidae) associated with the Japanese abalone Haliotis discus hannai and other native molluscs in Chile. Journal of Shellfish Research, 26(3): 863–867.
Bower, S. M., Blackbourn, J., Meyer, G. R., and Nishimura, D. J. H., 1992. Diseases of cultured Japanese scallops (Patinopecten Yessoensis) in British-Columbia, Canada. Aquaculture, 107(2–3): 201–210.
Eckelbarger, K. J., and Grassle, J. P., 1987. Spermatogenesis, sperm storage and comparative sperm morphology in nine species of Capitella, Capitomastus and Capitellides (Polychaeta: Capitellidae). Marine Biology, 95: 415–429.
Giangrande, A., 1997. Polychaeta reproductive patterns, life cycles and life histories: An overview. Oceanography and Marine Biology: An Annual Review, 35: 323–389.
Grassle, J. F., and Grassle, J. P., 1974. Opportunistic life histories and genetic systems in marine benthic polychaetes. Journal of Marine Research, 32: 253–284.
Hsieh, H., and Simon, J. L., 1990. The Sperm Transfer System in Kinbergonuphis simoni (Polychaeta: Onuphidae). The Biological Bulletin, 178: 85–93.
Jamieson, B. G. M., and Rouse, G. W., 1989. The spermatozoa of the Polychaeta (Annelida) — An ultrastructural review. Biological Reviews of the Cambridge Philosophical Society, 64(2): 93–157.
Jouin-Toulmond, C., and Purschke, G., 2004. Ultrastructure of the spermatozoa of Parenterodrilus taenioides (Protodrilida: ‘Polychaeta’) and its phylogenetic significance. Zoomorphology, 123: 139–146.
Lepore, E., Sciscioli, M., Mastrodonato, M., Gheradi, M., Giangrande, A., and Musco, L., 2006. Sperm ultra-structure and spermiogenesis in Syllis krohni (Polychaeta: Syllidae), with some observation on its reproductive biology. Scientia Marina, 70(4): 585–592.
Li, Q, Xu, K., and Yu, R., 2007. Genetic variation in Chinese hatchery populations of the Japanese scallop (Patinopecten yessoensis) inferred from microsatellite data. Aquaculture, 269(1–4): 211–219.
Lleonart, M., Handlinger, J., and Powell, M., 2003. Spionid mudworm infestation of farmed abalone (Haliotis spp.). Aquaculture, 221(1–4): 85–96.
Loosanoff, V. L., and Engle, J. B., 1943. Polydora in oysters suspended in, the water. Biological Bulletin, 85(1): 69–78.
Mcdiarmid, H., Day, R., and Wilson, R., 2004. The ecology of polychaetes that infest abalone shells in Victoria, Australia. Journal of Shellfish Research, 23(4): 1179–1188.
Mohammad, M. M., 1972. Infestation of pearl oyster Pinctada margaritifera (Linne) by a new species of Polydora in Kuwait, Arabian Gulf. Hydrobiologia, 39(4): 463–477.
Mortensen, S., Meeren, T. V., and Fosshagen, A., 2000. Mortality of scallop spat in cultivation, infested with tube dwelling bristle worm, Polydora sp. Aquaculture International, 8: 267–271.
Musco, L., Giangrande, A., Gherardi, M., Leopre, E., Mercurio, M., and Sciscioli, M., 2008. Sperm ultra-structure of Odontosyllis ctenostoma (Polychaeta: Syllidae) with inferences on syllid phylogeny and reproductive biology. Scientia Marina, 72(3): 421–427.
Nel, R., Coetzee, P. S., and Niekerk, G. V., 1996. The evaluation of two treatments to reduce mud worm (Polydora hoplura Claparede) infestation in commercially reared oysters (Crassostrea gigas Thunberg). Aquaculture, 141(1–2): 31–39.
Okoshi, S. W., and Okoshi, K., 1993. Microstructure of scallop and oyster shells infested with boring Polydora. Nippon Suisan Gakkaishi, 59(7): 1243–1247.
Purschke, G., and Fursman, M. C., 2005. Spermatogenesis and spermatozoa in Stygocapitella subterranea (Annelida, Parergodrilidae), an enigmatic supralittoral polychaete. Zoomorphology, 124: 137–148.
Read, G. B., 2010. Comparison and history of Polydora websteri and P. haswelli (Polychaeta: Spionidae) as mud-blister worms in New Zealand shellfish. New Zealand Journal of Marine and Freshwater Research, 44(2): 83–100.
Reunov, A. A., Yurchenko, O. V., Alexandrova, Y. N., and Radashevsky, V. I., 2009. Spermatogenesis in Boccardiella hamata (Polychaeta: Spionidae) from the Sea of Japan: Sperm formation mechanisms as characteristics for future taxonomic revision. Acta Zoologica, 91(4): 447–456.
Riascos, J. M., Heilmayer, O., Oliva, M. E., Laudien, J., and Arntz, W. E., 2008. Infestation of the surf clam Mescidesma donacium by the spionid polychaete Polydora bioccipitalis. Journal of Sea Research, 59(4): 217–227.
Rice, S. A., 1981. Spermatogenesis and sperm ultrastructure in three species of Polydora and in Streblospio benedicti (Polychaeta, Spionidae). Zoomorphology, 97(1–2): 1–16.
Rice, S. A., and Harrison, F. W., 1992. Polychaeta: Spermatogenesis and spermiogenesis. Microscopic Anatomy of Invertebrates, 7: 129–151.
Rice, S. A., Harrison, F. W., and Gardiner, S. L., 1982. Polychaeta: Spermatogenesis and spermiogenesis. Microscopic Anatomy of Invertebrates, 7: 129–151.
Rice, S. A., Karl, S., and Rice, K. A., 2008. The Polydora cornuta complex (Annelida: Polychaeta) contains populations that are reproductively isolated and genetically distinct. Invertebrate Biology, 127(1): 45–64.
Rouse, G. W., 1999. Polychaeta sperm: Phylogenetic and functional considerations. Hydrobiologia, 402: 215–224.
Sato-Okoshi, W., and Okoshi, K., 1993. Microstructure of scallop and oyster shells infested with boring Polydora. Nippon Suisan Gakkaishi, 59(7): 1243–1247.
Sato-Okoshi, W., Okoshi, K., Koh, B. S., Kim, Y. H., and Hong, J. S., 2012. Polydorid species (Polychaeta: Spionidae) associated with commercially important mollusk shells in Korean waters. Aquaculture, 350: 82–90.
Sato-Okoshi, W., Sugawara, Y., and Nomura, T., 1990. Reproduction of the boring polychaete Polydora-Variegata inhabiting scallops in Abashiri Bay, North Japan. Marine Biology, 104(1): 61–66.
Silina, A. V., 2006. Tumor-like formation on the shells of Japanese scallops Patinopecten yessoensis (Jay). Marine Biology, 148: 833–840.
Silina, A. V., and Zhukova, N. V., 2012. The benthic association between a bivalve and a shell boring polychaete and their potential food sources. Oceanology, 52(5): 646–654.
Simon, A. C., and Rouse, G. W., 2005. Ultrastructure of spermiogenesis, sperm, and the spermatheca in Terebrasabella heterouncinata (Polychaeta: Sabellidae: Sabellinae). Invertebrate Biology, 124(1): 39–49.
Simon, C. A., Kaiser, H., and Britz, P. J., 2004. Infestation of the abalone, Haliotis midae, by the sabellid, Terebrasabella heterouncinata, under intensive culture conditions, and the influence of infestation on abalone growth. Aquaculture, 232: 29–40.
Souto, V. S., Schejter, L., and Bremec, C. C., 2012. Epibionts on Aequipecten tehuelchus (d’Orbigny, 1846) (Pectinidae) in shelf waters off Buenos Aires, Argentina. American Malacological Bulletin, 30(2): 261–266.
Toulmond, J. C., and Purschke, G., 2004. Ultrastructure of the spermatozoa of Parenterodrilus taenioides (Protodrilida: ‘Polychaeta’) and its phylogenetic significance. Zoomorphology, 123: 139–146.
Tzetlin, A. B., Dahlgren, T., and Pueschke, G., 2002. Ultrastructure of the body wall, body cavity, nephridia and spermatozoa in four species of the Chrysopetalidae (Annelida, ‘Polychaeta’). Zoologischer Anzeiger, 241: 37–55.
Walker, L. M., 2011. A review of the current status of the Polydora-complex (Polychaeta: Spionidae) in Australia and a checklist of recorded species. Zootaxa, 2751: 40–62.
Wilson, W. H., 1991. Sexual reproductive modes in polychaetes: Classification and diversity. Bulletin of Marine Science, 48(2): 500–516.
Zhu, J. Q., Dahms, H. U., and Yang, W. X., 2008. Ultrastructure of the mature spermatozoon of the bivalve Scapharca broughtoni (Mollusca: Bivalvia: Arcidae). Micron, 39(8): 1205–1209.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, Y., Zhang, T., Zhang, L. et al. Ultrastructure developments during spermiogenesis in Polydora ciliata (Annelida: Spionidae), a parasite of mollusca. J. Ocean Univ. China 13, 1071–1077 (2014). https://doi.org/10.1007/s11802-014-2309-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-014-2309-y