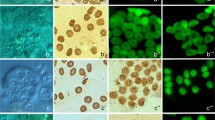
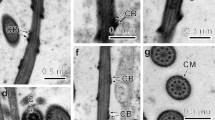
Abstract
Spermatogenesis and spermatozoa ultrastructure of the amphibian leech Batracobdella algira Moquin-Tandon, 1846 (Hirudinida: Glossiphoniidae) have been investigated by means of electron and fluorescent microscopy. In B. algira, there are seven pairs of testisacs (testes) that are located latero-ventrally throughout the body. Each testis contains numerous cysts with developing germ cells. The germ cells in a given cyst are in the same developmental stage (i.e., there are spermatogonial, spermatocytic, and spermatid cysts); however, there is no developmental synchrony between the cysts, and therefore, all of the developmental stages occur simultaneously in the same testis. In the cysts, each germ cell is connected to acentral cytoplasmic mass, the cytophore, by one intercellular bridge. The spermatozoa of the studied species conform to the general organization plan that is known for Hirudinida: they are filiform cells that are formed in sequence by an elongated and twisted acrosome that consists of an anterior and posterior acrosome, a fully condensed and helicoid nucleus, a midpiece composed of a single and twisted mitochondrion that is characteristically surrounded by an electron-dense sheath, and a flagellum with the conventional 9 × 2 + 2 axonemal pattern. Using a comprehensive approach, we compared our findings with the ultrastructural data that had been obtained from the spermatozoa of the other hirudinids that have been studied to date. Only minor differences in the length and shape of the studied organelles were found which seems to be connected with the different ways of insemination, specific properties of female reproductive tracts, and physiology of fertilization. Additionally, we studied the organization of the microtubular cytoskeleton in male germline cysts at consecutive stages of spermatogenesis using fluorescent and electron microscopy. By comparing the present data with those from Oligochaeta, Branchiobdellida, and Acanthobdellida, we found that only the presence of an anterior acrosome characterizes the true leeches and that, at present, should be regarded as an autapomorphic character of Hirudinida. Our results showed that the arrangement of the microtubules changed dynamically during spermatogenesis.









Similar content being viewed by others
References
Arcidiacono G (1979) Differentiation of the Batracobdella paludosa sperm cell. Acta Embryologiae Experimentalis 2:209–228
Barlan K, Gelfand V (2017) Microtubule-based transport and the distribution, tethering, and organization of organelles. Cold Spring Harb Perspect Biol 9(5):a025817. https://doi.org/10.1101/cshperspect.a025817
Ben Ahmed R, Tekaya S (2009) Etat des connaissances sur la Biogéographie des Hirudinées du pourtour de la Méditérranée. Bull Soc Zool Fr 134:99–124
Ben Ahmed R, Ben Rached B, Tekaya S (2014) Observations on leech parasitism and predation for Tunisian amphibians. Lauterbornia 77:1–7
Ben Ahmed R, Bacchetta R, Boesi R, Froman N, Marotta R, Ferraguti M (2015a) The spermatozoa of Hirudinea with examples from three different taxa. Zool Anz 255:54–61
Ben Ahmed R, Tekaya S, Urbisz AZ, Świątek P (2015b) Ultrastructural study of spermatogenesis and sperm in the African medicinal leech Hirudo troctina Johnson, 1816 (Annelida, Hirudinida). Tissue Cell 47:242–253
Bonet S, Molinas M (1988) Ultrastructure of the sperm and spermatogenesis and spermiogenesis of Dina lineata (Hirudinea, Erpobdellidae). Gamete Research 19:177–190
Bonet S, Molinas M, Garcia-Mas I (1988) Testicular spermatozoa and male germ cells evolution in Dina lineata (Hirudinea, Erpobdellidae) studied by scanning electron microscopy. Butlletí de la Institució Catalana d'Història Natural 55:17–25
Cardini A, Ferraguti M (2004) The phylogeny of Branchiobdellida (Annelida, Clitellata) assessed by sperm characters. Zool Anz 243:37–46
Damas D (1968) Origine et structure du spermatophore de Glossiphonia complanata (L.) (Hirudinée, Rhynchobdelle). Archive de Zoologie Expérimentale 109:79–85
Erséus C, Källersjö M (2004) 18S rDNA phylogeny of Clitellata (Annelida). Zool Scr 33:187–196
Fernández J, Tellez V, Olea N (1992) Hirudinea. In: Harrison FW, Gardiner SL (eds) Microscopic anatomy of invertebrates, VII edn. Wiley-Liss, New York, pp 323–394
Ferraguti M (2000) In: Adiyodi, K.G., Adiyodi, R.G. (Eds.), Clitellata in reproductive biology of invertebrates, Vol. 9, Part B, Progress in male gamete ultrastructure and phylogeny. John Wiley and Sons, New Delhi, Calcutta, Oxford, pp. 125–182
Ferraguti M, Erséus C (1999) Sperm types and their use for a phylogenetic analysis of aquatic clitellates. Hydrobiologia 402:225–237
Ferraguti M, Lanzavecchia G (1971) Morphogenetic effects of microtubules. I. Spermiogenesis in Annelida Tubificidae. J Submicrosc Cytol 3:121–137
Fishman EL, Jo K, Nguyen QPH, Kong D, Royfman R, Cekic AR, Khanal S, Miller AL, Simerly C, Schatten G, Loncarek J, Mennella V, Avidor-Reiss T (2018) A novel atypical sperm centriole is functional during human fertilization. Nat Commun 9(1):2210. https://doi.org/10.1038/s41467-018-04678-8
Franzén Å (1977) Gametogenesis of bryozoans. In: Woollacott RM, Zimmer RL (eds) Biology of bryozoans. Academic Press, New York, pp 1–22
Franzén Å (1991) Spermiogenesis and sperm ultrastructure in Acanthobdella peledina (Hirudinea) with some phylogenetic considerations. Invertebr Reprod Dev 19:245–256
Garavaglia C, Lora Lamia Donin C, Lanzavecchia G (1974) Ultrastructural morphology of spermatozoa of Hirudinea. J Submicrosc Cytol 6:229–244
Gouda HA (2013) Spermatogenesis, spermiogenesis and sperm transfer in two freshwater leeches from Assiut, Egypt. Res Zool 3:45–55
Govedich FR, Moser WE (2015) Clitellata: Hirudinida and Acanthobdellida. Pp. 565-588, In: Thorp, J.H.; Rogers, D.C. (Eds.) Ecology and general biology. Thorp and Covich's freshwater invertebrates volume 1. 4th Edition
Greenbaum MP, Iwamori T, Buchold GM, Matzuk MM (2011) Germ cell intercellular bridges. Cold Spring Harb Perspect Biol 3:1–18
Grosser C, Pešić V (2006) On the diversity of Iranian leeches (Annelida: Hirudinea). Archives of Biological Science of Belgrade 58:21–24
Haglund K, Nezis IP, Stenmark H (2011) Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun Integr Biol 4:1–9
Jamieson BGM (1981) The ultrastructure of the Oligochaeta. Academic Press, London, New York, Toronto, Sydney, San Francisco, pp. 462
Jamieson BGM (1992) Oligochaeta. In: F. W. Harrison & S. L. Gardiner (eds), Microscopic anatomy of invertebrates, vol. 7: Annelida. Wiley-Liss, New York, pp. 217–322
Jamieson BGM (2006) Non-leech clitellata. In: Rouse, G., Pleijel, F. (Eds.), Reproductive biology and phylogeny of Annelida, Enfield (NH). Science Publishers, Jersey, Plymouth, pp. 235–392
Jamieson BGM, Erséus C, Ferraguti M (1987) Parsimony analysis of the phylogeny of some Oligochaeta (Annelida) using spermatozoal ultrastructure. Cladistics 3:145–155
Kholmukhamedov A, Schwartz JM, Lemasters JJ (2013) MitoTracker probes and mitochondrial membrane potential. Shock 39:543
Lehti MS, Sironen A (2016) Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 151:R43–R54. https://doi.org/10.1530/REP-15-0310
Lora Lamia Donin C, Lanzavecchia G (1974) Morphogenetic effects of microtubules. III. Spermiogenesis in annelid Hirudinea. J Submicrosc Cytol 6:245–259
Lu K, Jensen L, Lei L, Yamashita YM (2017) Stay connected: a germ cell strategy. Trends Genet 33:971–978
Lukin EI (1962) Fauna Ukraini. Pijavki. Institute Zoology Akademie Nauk Ukraïns. RSR, 30:1–196, Kiew
Lukin EI (1976) Fauna USSR, Pijavki. Akademie der Wissenschaften der USSR, Vol. 1, Moskau
Malécha J (1975) Étude ultrastructurale de la spermiogénèse de Piscicola geometra (Hirudinée, Rhynchobdelle). J Ultrastruct Res 51:188–203
Małota K, Świątek P (2016) Analysis of the cytoskeleton organization and its possible functions in male earthworm germ-line cysts equipped with a cytophore. Cell Tissue Res 366:175–189
Marotta R, Ferraguti M (2009) Sperm ultrastructure in assessing phylogenetic relationships among clitellate annelids. In: Shain DH (ed) Annelids in modern diology. Wiley-Blackwell, Hoboken, NJ, pp 314–327
Marotta R, Ferraguti M, Erséus C, Gustavsson LM (2008) Combined-data phylogenetics and character evolution of Clitellata (Annelida) using 18S rDNA and morphology. Zool J Linnean Soc 154:1–26
Marotta R, Ferraguti M, Erséus C (2003) A phylogenetic analysis of Tubificinae and Limnodriloidinae (Annelida Clitellata, Tubificidae) using sperm and somatic characters. Zool Scr 32:255–278
Martin P (2001) On the origin of the Hirudinea and the demise of the Oligochaeta. Proc R Soc Lond B Biol Sci 268:1089–1098
Martinucci GB, Felluga B, Carli S (1977) Development and degeneration of Cytophorus during spermiohistogenesis in Eisenia foetida (Sav). Boll Zool 44:383–398
McHugh D (1997) Molecular evidence that echiurans and pogonophorans are derived annelids. Proc Natl Acad Sci 94:8006–8009
Nielsen C (1995) Animal evolution: interrelationships of the animal phyla. Oxford University Press
Pastisson C (1977) L’ultrastructure des cellules séminales de la sangsue Hirudo medicinalis au cours de leur différenciation. Annales des Sciences Naturelles – Zoologie Paris 19:315–347
Pepling ME, de CM, Spradling AC (1999) Germline cysts: a conserved phase of germ cells development? Trends Cell Biol 9:257–262
Purschke G, Westheide W, Rohde D, Brinkhurst RO (1993) Morphological reinvestigation and phylogenetic relationship of Acanthobdella peledina (Annelida, Clitellata). Zoomorphology 113:91–101
Rice SA (1992) Polychaeta: spermatogenesis and spermiogenesis. In Harrison, F. W. and Gardiner, S. L. (Eds): Microscopic anatomy of invertebrates. Volume 7: Annelida, pp. 129–151. Wiley-Liss, Inc., New York
Romdhane Y, Ben Ahmed R, Tekaya S (2014) Insemination and embryonic development of the Glossiphoniidae leech: Batracobdella algira (Annelida, Clitellata). Invertebr Reprod Dev 59:17–25
Russell LD, Russell JA, MacGregor GR, Meistrich ML (1991) Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat 192:97–120
Sawyer RT (1986) Leech biology and behaviour. In: Feeding biology, ecology, and systematics, 2nd edn. Clarendon Press, Oxford, UK
Siddall ME, Apakupakul K, Burreson EM, Coates KA, Erséus C, Gelder SR, Källersjö M, Trapido-Rosenthal H (2001) Validating Livanow: molecular data agree that leeches, Branchiobdellidans, and Acanthobdella peledina form a monophyletic group of oligochaetes. Mol Phylogenet Evol 21:346–351
Struck TH, Paul C, Hill N, Hartmann S, Hösel C, Kube M, Lieb B, Meyer A, Tiedemann R, Purschke G, Bleidorn C (2011) Phylogenomic analyses unravel annelid evolution. Nature. 471(7336):95–98
Struck TH, Schult N, Kusen T, Hickman E, Bleidorn C, McHugh D, Halanych KM (2007) Annelid phylogeny and the status of Sipuncula and Echiura. BMC Evol Biol 7(1):57
Światek P, Kubrakiewicz J, Klag J (2009) Formation of germ-line cysts with a central cytoplasmic core is accompanied by specific orientation of mitotic spindles and partitioning of existing intercellular bridges. Cell Tissue Res 337:137–148
Tessler M, de Carle D, Voiklis ML, Gresham OA, Neumann JS, Cios S, Siddall ME (2018) Worms that suck: phylogenetic analysis of Hirudinea solidifies the position of Acanthobdellida and necessitates the dissolution of Rhynchobdellida. Mol Phylogenet Evol 127:129–134
Westheide W, Purschke G (1996) Proacrosome and acrosome of the spermatozoon in Acanthobdella peledina (Annelida: Clitellata). Invertebr Reprod Dev 29:223–230
Westheide W, Purschke G, Middendorf K (1991) Spermatozoal ultrastructure of the taxon Enchytraeus (Annelida, Oligochaeta) and its significance for species discrimination and identification1, 2. J Zool Syst Evol Res 29:323–342
Wissocq JCL, Malécha J (1975) Étude des spermatozoïdes d’hirudinées à l’aide de la technique de coloration negative. J Ultrastruct Res 52:340–361
Yang J, Dungrawala H, Hua H, Manukyan A, Abraham L, Lane W, Mead H, Wright J, Schneider BL (2011) Cell size and growth rate are major determinants of replicative lifespan. Cell Cycle 10:144–155
Funding
R.B.A. would like to thank the financial support of The Tunisian Ministry of High Education and Scientific Research (UR18ES41). The work was supported by funds for statutory activities of the Faculty of Biology and Environmental Protection, University of Silesia in Katowice. We cordially thank the Reviewers, whose helpful and sincere comments have improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Georg Krohne
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research Highlights:
The results that were obtained show that:
1. The species studied has the general features of hirudinid spermatozoa,
2. Interspecific variations were found in nearly all of the characters that were examined compared to B. paludosa and other Hirudinida.
3. By comparing the present data with those from Oligochaeta, Branchiobdellida, and Acanthobdellida, we found that only the presence of an anterior acrosome characterizes the true leeches and that, at present, should be regarded as an autapomorphic character of Hirudinida.
4. The distribution of the MTs changes during the consecutive stages of spermatogenesis; during early spermatogenesis, they seem to be engaged in the transfer of the cytoplasm from the cells towards the cytophore, and after which, they form the manchette and the axoneme.
Rights and permissions
About this article
Cite this article
Ahmed, R.B., Malota, K., Jarosz, N. et al. Microscopic analysis of spermatogenesis and mature spermatozoa in the amphibian leech Batracobdella algira (Annelida, Clitellata, Hirudinida). Protoplasma 256, 1609–1627 (2019). https://doi.org/10.1007/s00709-019-01407-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01407-w