Abstract
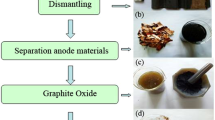
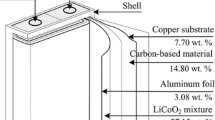
With extensive use of lithium ion batteries (LIBs), amounts of LIBs were discarded, giving rise to growth of resources demand and environmental risk. In view of wide usage of natural graphite and the high content (12%–21%) of anode graphite in spent LIBs, recycling anode graphite from spent LIBs cannot only alleviate the shortage of natural graphite, but also promote the sustainable development of related industries. After calcined at 600°C for 1 h to remove organic substances, anode graphite was used to prepare graphene by oxidation-reduction method. Effect of pH and N2H4·H2O amount on reduction of graphite oxide were probed. Structure of graphite, graphite oxide and graphene were characterized by XRD, Raman and FTIR. Graphite oxide could be completely reduced to graphene at pH 11 and 0.25 mL N2H4·H2O. Due to the presence of some oxygen-containing groups and structure defects in anode graphite, concentrated H2SO4 and KMnO4 consumptions were 40% and around 28.6% less than graphene preparation from natural graphite, respectively.

Similar content being viewed by others
References
Suzuki T, Nakamura T, Inoue Y, Niinae M, Shibata J. A hydrometallurgical process for the separation of aluminum, cobalt, copper and lithium in acidic sulfate media. Separation and Purification Technology, 2012, 98: 396–401
Zou H, Gratz E, Apelian D, Wang Y. A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chemistry, 2013, 15(5): 1183
Zeng X, Li J, Ren Y. Prediction of various discarded lithium batteries in China. IEEE International Symposium on Sustainable Systems and Technology, 2012
Li J, Wang G, Xu Z. Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries. Waste Management (New York, N.Y.), 2016, 52: 221–227
Gratz E, Sa Q, Apelian D, Wang Y. A closed loop process for recycling spent lithium ion batteries. Journal of Power Sources, 2014, 262: 255–262
Ferreira D A, Prados L M Z, Majuste D, Mansur M B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. Journal of Power Sources, 2009, 187(1): 238–246
He L P, Sun S Y, Song X F, Yu J G. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Management (New York, N.Y.), 2015, 46: 523–528
Xin Y Y, Guo X M, Chen S, Wang J, Wu F, Xin B. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. Journal of Cleaner Production, 2016, 116: 249–258
Wang X, Gaustad G, Babbitt C W. Targeting high value metals in lithium-ion battery recycling via shredding and size-based separation. Waste Management (New York, N.Y.), 2016, 51: 204–213
Joo S H, Shin D J, Oh C H, Wang J P, Senanayake G, Shin S M. Selective extraction and separation of nickel from cobalt, manganese and lithium in pre-treated leach liquors of ternary cathode material of spent lithium-ion batteries using synergism caused by Versatic 10 acid and LIX 84-I. Hydrometallurgy, 2016, 159: 65–74
Sun Z, Cao H, Xiao Y, Sietsma J, Jin W, Agterhuis H, Yang Y. Toward sustainability for recovery of critical metals from electronic waste: The hydrochemistry processes. ACS Sustainable Chemistry & Engineering, 2017, 5(1): 21–40
Zheng X, Gao W, Zhang X, He M, Lin X, Cao H, Zhang Y, Sun Z. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Management (New York, N.Y.), 2017, 60: 680–688
Moradi B, Botte G G. Recycling of graphite anodes for the next generation of lithium ion batteries. Journal of Applied Electrochemistry, 2016, 46(2): 123–148
Sur U K, Saha A, Datta A, Ankamwar B, Surti F, Roy S D, Roy D. Synthesis and characterization of stable aqueous dispersions of graphene. Bulletin of Materials Science, 2016, 39(1): 159–165
Singh C, Ali M A, Sumana G. Green synthesis of graphene based biomaterial using fenugreek seeds for lipid detection. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 871–880
Du Y L, Lei X L, Zhang F L. Analysis on the development of graphite and recommended management strategies. China Mining Magazing, 2015, 24: 28–29 (in Chinese)
Gao T M, Chen Q S, Yu W J, Shen L. Projection of Chinas graphite demand and development prospects. Resources Science, 2015, 37 (5): 1059–1067 (in Chinese)
Dao T D, Jeong H M. Graphene prepared by thermal reduction–exfoliation of graphite oxide: Effect of raw graphite particle size on the properties of graphite oxide and graphene. Materials Research Bulletin, 2015, 70: 651–657
Guo Y, Li F, Zhu H, Li G, Huang J, He W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Management (New York, N.Y.), 2016, 51: 227–233
Roy I, Sarkar G, Mondal S, Rana D, Bhattacharyya A, Saha N R, Adhikari A, Khastgir D, Chattopadhyay S, Chattopadhyay D. Synthesis and characterization of graphene from waste dry cell battery for electronic applications. RSC Advances, 2016, 6(13): 10557–10564
Yu H, Zhang B, Bulin C, Li R, Xing R. High-efficient synthesis of graphene oxide based on improved hummers method. Scientific Reports, 2016, 6(1): 36143
Wang R Y, Wu Z W, Qin Z F, Chen C, Zhu H, Wu J, Chen G, Fan W, Wang J. Graphene oxide: An effective acid catalyst for the synthesis of polyoxymethylene dimethyl ethers from methanol and trioxymethylene. Catalysis Science & Technology, 2016, 6(4): 993–997
Yusof N S, Babgi B, Alghamdi Y, Aksu M, Madhavan J, Ashokkumar M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrasonics Sonochemistry, 2016, 29: 568–576
Stankovich S, Dikin D A, Piner R D, Kohlhaas K A, Kleinhammes A, Jia Y, Wu Y, Nguyen S B T, Ruoff R S. Synthesis of graphenebased nanosheets via chemical reduction of exfoliated graphite oxide. Carbon, 2007, 45(7): 1558–1565
Rahimi R, Moshari M, Rabbani M, Azad A. Photooxidation of benzyl alcohols and photodegradation of cationic dyes by Fe3O4 sulfur/reduced graphene oxide as catalyst. RSC Advances, 2016, 6 (47): 41156–41164
QIAO H. Study on the structural transformation and electrical properties of products formed by the oxidation-reduction of graphite. Dissertation for Doctor’s Degree. Chongqing: Southwest Univerisity, 2012
Li D, Müller M B, Gilje S, Kaner R B, Wallace G G. Processable aqueous dispersions of graphene nanosheets. Nature Nanotechnology, 2008, 3(2): 101–105
Soltani T, Lee B K. Mechanism of highly efficient adsorption of 2-chlorophenol onto ultrasonic graphene materials: Comparison and equilibrium. Journal of Colloid and Interface Science, 2016, 481: 168–180
Acknowledgements
This work is supported by the Key Research Project of Science and Technology Commission of Shanghai Municipality (No. 12dz2294000). The authors would like to thank the anonymous reviewers for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Liu, Z., Xia, J. et al. Preparing graphene from anode graphite of spent lithium-ion batteries. Front. Environ. Sci. Eng. 11, 6 (2017). https://doi.org/10.1007/s11783-017-0993-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-017-0993-8