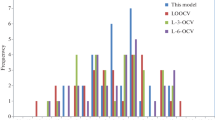
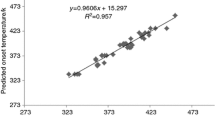
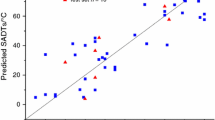
Abstract
The thermal decomposition temperature is one of the most important parameters to evaluate fire hazard of organic peroxide. A quantitative structure-property relationship model was proposed for estimating the thermal decomposition temperatures of organic peroxides. The entire set of 38 organic peroxides was at random divided into a training set for model development and a prediction set for external model validation. The novel local molecular descriptors of AT1, AT2, AT3, AT4, AT5, AT6 and global molecular descriptor of ATC have been proposed in order to character organic peroxides’ molecular structures. An accurate quantitative structure-property relationship (QSPR) equation is developed for the thermal decomposition temperatures of organic peroxides. The statistical results showed that the QSPR model was obtained using the multiple linear regression (MLR) method with correlation coefficient (R), standard deviation (S), leave-one-out validation correlation coefficient (RCV) values of 0.9795, 6.5676 °C and 0.9328, respectively. The average absolute relative deviation (AARD) is only 3.86% for the experimental values. Model test by internal leave-one-out cross validation and external validation and molecular descriptor interpretation were discussed. Comparison with literature results demonstrated that novel local and global descriptors were useful molecular descriptors for predicting the thermal decomposition temperatures of organic peroxides.
摘要
热分解温度是评估有机过氧化物火灾危险程度最重要的参数之一。本文提出一种估算有机过氧 化物热分解温度的定量新方法。38 种有机过氧化物被随机分为训练集和测试集, 分子局部描述符AT1, AT2, AT3, AT4, AT5, AT6 和全局描述符ATC 表征分子结构特征。建立了一个准确的估算有机过氧化物热 分解温度的定量构效关系模型, 多元线性关系模型的相关系数、标准偏差和留一法检验的相关系数分 别为0.9795, 6.5676 °C 和 0.9328, 预测结果的平均相对误差仅为3.86%。模型稳定性采用留一法和外 检验进行验证, 分子结构参数对有机过氧化物的热分解温度的影响进行合理的解释。与相关文献结果 比较表明利用分子局部描述符AT1, AT2, AT3, AT4, AT5, AT6 和全局描述符ATC 建立定量构效关系方法估 算有机过氧化物的热分解温度是一种有效的方法。
Similar content being viewed by others
References
HERBERT K, PETER H G., RAINER S, WILFIRED M. Peroxy compounds, organic in Ullmann’s encyclopedia of industrial chemistry [M]. New York: Wiley-VCH, Weinheim, 2002.
SANCHEZ J, MYERS T. Peroxides and peroxide compounds organic peroxides [M]//Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed, New York: John Wiley & Sons, 1996: 230–310.
ZHANG W, XIAO J, WANG X, MIAO G, YE F Y, LI Z. Oxidative desulfurization using in-situ-generated peroxides in diesel by light irradiation [J]. Energy Fuels, 2014, 28: 5339–5344.
SAULE M, MOINE L, DEGUEIL-CASTAING M, MAILLARD B. Chemical modification of polypropylene by decomposition of unsaturated peroxides [J]. Macromolecules, 2005, 38: 77–85.
TOMMASO S D I, ROTUREAU P, CRESCENZI O, ADAMO C. Oxidation mechanism of diethyl ether: A complex process for a simple molecule [J]. Phys Chem Chem Phys, 2011, 13: 14636–14645.
BENASSI R, TADDEI F. Homolytic bond-dissociation in peroxides, peroxyacids, peroxyesters and related radicals: ab-initio MO calculations [J]. Tetrahedron, 1994, 50: 4795–4810.
SHEN S J, WU S H, CHI J H, WANG Y W, SHU C M. Thermal explosion simulation and incompatible reaction of dicumyl peroxide by calorimetric technique [J]. J Therm Anal Calorim, 2010, 102: 569–577.
CSB. Improving reactive hazard management [R]. Washington, DC, 2002.
HSU J M, SU M S, HUANG C Y, DUH Y S. Calorimetric studies and lessons on fires and explosions of a chemical plant producing CHP and DCPO [J]. J Hazard Mater, 2012, 217–218: 19–28.
TSENG J M, LIN C P. Green thermal analysis technology for evaluating the thermal hazard of di-tert-butyl peroxide [J]. Ind Eng Chem Res, 2011, 50: 9487–9494.
SWIHART M T, GIRSHICK S L. Thermochemistry and kinetics of silicon hydride cluster formation during thermal decomposition of silane [J]. J Phys Chem B, 1998, 103: 64–76.
DUH Y S, WU X H, KAO C S. Hazard ratings for organic peroxides [J]. Proc Safety Prog, 2008, 27: 89–99.
LV J Y, CHEN W H, CHEN L P, TIAN Y T, YAN J J. Thermal risk evaluation on decomposition processes for four organic peroxides [J]. Thermochim Acta, 2004, 589: 11–18.
VIDAL M, ROGERS W J, HOLSTE J C, MANNAN M S. A review of estimation methods for flash points and flammability limits [J]. Proc Safety Prog, 2004, 23: 47–55.
WANG Q, WANG L, ZHANG X, MI Z. Thermal stability and kinetic of decomposition of nitrated HTPB [J]. J Hazard Mater, 2009, 172: 1659–1664.
LV J, CHEN L, CHEN W, GAO H, PENG M. Kinetic analysis and self-accelerating decomposition temperature of dicumyl peroxide [J]. Thermochim Acta, 2013, 571: 60–63.
YEH P Y, SHU C M, DUH Y S. Thermal hazard analysis of methyl ethyl ketone peroxide [J]. Ind Eng Chem Res, 2002, 42: 1–5.
MALOW M, WEHRSTEDT K D. Prediction of the self-accelerating decomposition temperature for liquid organic peroxides from differential scanning calorimetry (DSC) measurements [J]. J Hazard Mater, 2005, 120: 21–24.
ALBAHRI T A. Flammability characteristics of pure hydrocarbons [J]. Chem Eng Sci, 2003, 58: 3629–3641.
PRANA V, ROTUREAU P, FAYET G, ANDRE D, HUB S, VICOT P, RAO L, ADAMO C. Prediction of the thermal decomposition of organic peroxides by validated QSPR models [J]. J Hazard Mater, 2014, 276: 216–224.
LU Y, NG D, MANNAN M S. Prediction of the reactivity hazards for organic peroxides using QSPR approach [J]. Ind Eng Chem Res, 2011, 50: 1515–1522.
ASTM Computer Program for Chemical Thermodynamic and Energy Release Evaluation-CHETAH [EB/OL. [2014–01]. https://doi.org/www.astm.org.
MOHAN V K, BECKER K R, HAY J E. Hazard evaluation of organic peroxides [J]. J Hazard Mater, 1982, 5: 197–220
GHARAGHEIZI F, SATTARI M, ILANI-KASHKOULI P, MOHAMMADI A H, RAMJUGERNATH D, RICHON D. Quantitative structure-property relationship for thermal decomposition temperature of ionic liquids [J]. Chem Eng Sci, 2012, 84: 557–563.
PAN Y, ZHANG Y Y, JIANG J C, DING L. Prediction of the self-accelerating decomposition temperature of organic peroxides using the quantitative structure-property relationship (QSPR) approach [J]. J Loss Prev Process Ind, 2014, 31: 41–49.
KATRITZKY A R, KUANAR M, SLAVOV S, HALL C D, KARELSON M, KAHN I, DOBCHEV D A. Quantitative correlation of physical and chemical properties with chemical structure: utility for prediction [J]. Chem Rev, 2010, 110: 5714–5789.
TROPSHA A, GOLBRAIKH A. Predictive QSAR modeling workflow, model applicability domains, and virtual screening [J]. Curr Pharm Des, 2007, 13: 3494–3504.
GHARAGHEIZI F, ESLAMIMANESH A, MOHAMMADI A H, RICHON D. QSPR approach for determination of parachor of non-electrolyte organic compounds [J]. Chem Eng Sci, 2011, 66: 2959–2967.
DAI Y M, LIU H, LI X, ZHU Z P, ZHANG Y F, CAO Z, ZHU L X, ZHOU Y. A novel group contribution-based method for estimation of flash points of ester compounds [J]. Chemom Intell Lab Syst, 2014, 136: 138–146.
JIN L J, BAI P. Prediction of the normal boiling point of oxygen containing organic compounds using quantitative structure-property relationship strategy [J]. Fluid Phase Equilibria, 2016, 427: 194–201.
XU J, WANG L, WANG L X, LIANG G J, SHEN X L, XU W L. Prediction of Setschenow constants of organic compounds based on a 3D structure representation [J]. Chemom Intell Lab Syst, 2011, 107: 178–184.
VENKATRAMAN V, ALSBERG B K. Quantitative structure-property relationship modeling of thermal decomposition temperatures of ionic liquids [J]. J Mol Liq, 2016, 223: 60–67.
ASTM E537-02. Standard Test Method for the Thermal Stability of Chemicals by Differential Scanning Calorimetry [S].
DAI Y M, ZHU Z P, CAO Z, ZHANG Y F, LI X. Prediction of boiling points of organic compounds by QSPR tools [J]. J Mol Graphics Model, 2013, 44: 113–119.
DAI Y M, LIU H, NIU L L, CHEN C, CHEN X Q, LIU Y N. Estimation of half-wave potential of anabolic androgenic steroids by means of QSER approach [J]. Journal of Central South University, 2016, 23: 1906–1914.
ZHOU C, CHU X, NIE C. Predicting thermodynamic properties with a novel semi-empirical topological descriptor and path numbers [J]. J Phy Chem B, 2007, 111: 10174–10179.
DUCHOWICZ P R, CASTRO E A, FERNÁ NDEZ F M, GONZALEZ M P. A new search algorithm for QSPR/QSAR theories: Normal boiling points of some organic molecules [J]. Chem Phys Lett, 2005, 412: 376–380.
ROY K, CHAKRABORTY P, MITRA I, OJHA P K, KAR S, DAS R N. Some case studies on application of “r2m” metrics for judging quality of quantitative structure activity relationship predictions: Emphasis on scaling of response data [J]. J Comput Chem, 2013, 34: 1071–1082.
DAI Yi, LIU You, LI Xun, CAO Zhong, ZHU Zhi, YANG Dao. Estimation of surface tension of organic compounds using QSPR [J]. Journal of Central South University, 2012, 19(1): 93–100.
JIN L J, BAI P. QSPR study on normal boiling point of acyclic oxygen containing organic compounds by radial basis function artificial neural network [J]. Chemom Intell Lab Syst, 2016, 157: 127–132.
DAI Yi, HUANG Ke, LI Xun, CAO Zhong, ZHU Zhi, YANG Dao. Simulation of 13C NMR chemical shifts of carbinol carbon atoms by using quantitative structure-spectrum relationships [J]. Journal of Central South University of Technology, 2011, 18(2): 323–340.
SHI J J, CHEN L P, CHEN W H, SHI N, YANG H, XU W. Prediction of the thermal conductivity of organic compounds using heuristic and support vector machine methods [J]. Acta Phys Chem Sina, 2012, 28: 2790–2796.
PAN Y, JIANG J C, WANG R, CAO H Y, CUI Y. A novel QSPR model for prediction of lower flammability limits of organic compounds based on support vector machine [J]. J Hazard Mater, 2009, 168: 962–969.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2015SK20823) supported by Science and Technology Project of Hunan Province, China; Project(15A001) supported by Scientific Research Fund of Hunan Provincial Education Department, China; Project(2017CL06) supported by Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, China; Project(k1403029-11) supported by Science and Technology Project of Changsha City, China; Project(CX2015B372) supported by the Hunan Provincial Innovation Foundation for Postgraduate, China
Rights and permissions
About this article
Cite this article
Dai, Ym., Niu, Ll., Zou, Jq. et al. Estimation of thermal decomposition temperatures of organic peroxides by means of novel local and global descriptors. J. Cent. South Univ. 25, 1535–1544 (2018). https://doi.org/10.1007/s11771-018-3846-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-018-3846-0
Key words
- organic peroxide
- thermal decomposition temperature
- multiple linear regression
- model validation
- quantitative structure-property relationship